| Patient ID | Sex | Diagnosis | Age | Tumor Stage | Tumor grade | Disease Status | Relapse Type | Survival Status | Survival Time | Missense Mutation - KRAS | Missense Mutation - TP53 | Missense Mutation - CDKN2A | Missense Mutation - SMAD4 |
|---|
Patient ID | Sex | Diagnosis | Age | Tumor Stage | Tumor grade | Disease Status | Relapse Type | Survival Status | Survival Time | Missense Mutation - KRAS | Missense Mutation - TP53 | Missense Mutation - CDKN2A | Missense Mutation - SMAD4 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| DO221539 | Male | Adenocarcinoma, NOS | 49.0 | IIB | G2 | Relapse | Local recurrence | Alive | 1733.0 | G12R | R282W,R150W | No | No |
| DO221540 | Male | Adenocarcinoma, NOS | 56.0 | IIB | G3 | NA | NA | Deceased | 290.0 | G12D | No | No | No |
| DO221541 | Male | PDAC | 75.0 | IIA | G2 | Complete remission | NA | Deceased | 195.0 | G12D | No | No | No |
| DO221542 | Male | Carcinoma, NOS | 60.0 | IIA | G4 | Complete remission | NA | Alive | 375.0 | G12D | R141H,R273H | No | No |
| DO221543 | Male | Adenocarcinoma, NOS | 54.0 | IIB | G3 | Complete remission | NA | Alive | 2045.0 | G12R | No | No | No |
| DO221544 | Male | PDAC | 42.0 | IV | NA | Complete remission | NA | Alive | 467.0 | Q61L | No | No | No |
| DO221545 | Male | PDAC | 63.0 | IB | G2 | Complete remission | NA | Alive | 260.0 | G12D | No | No | No |
| DO221546 | Male | PDAC | 61.0 | IIB | G3 | NA | NA | Deceased | 480.0 | G12D | No | No | No |
| DO221547 | NA | PDAC | 47.0 | IV | G2 | Progression | Distant recurrence/metastasis | Alive | 1338.0 | G12D | No | No | No |
| DO224575 | Male | PDAC | 72.0 | IIB | G2 | No evidence of disease | NA | Deceased | 826.0 | G12D | S127F,S34F | No | No |
| DO224596 | Male | PDAC | 60.0 | IIB | G2 | Relapse | Local recurrence | Deceased | 819.0 | G12D | No | No | No |
| DO224633 | Male | PDAC | 72.0 | IIB | G3 | Relapse | Distant recurrence/metastasis | Deceased | 742.0 | No | No | No | No |
| DO224642 | Female | PDAC | 57.0 | IIA | G3 | NA | NA | Deceased | 547.0 | G12D | R141H,R273H | No | No |
| DO224648 | Male | PDAC | 69.0 | IIB | G3 | Relapse | Distant recurrence/metastasis | Deceased | 958.0 | G12R | R248Q,R155Q,R116Q | No | No |
| DO224656 | Female | PDAC | 50.0 | IIB | G2 | Relapse | Local recurrence and distant metastasis | Deceased | 1208.0 | G12R | R213L,R120L,R81L | No | No |
| DO224688 | Male | PDAC | 61.0 | IIB | G3 | Relapse | Local recurrence | Deceased | 633.0 | G12R | V272L,V140L | No | No |
| DO224698 | Male | IPMN | 72.0 | IIB | G3 | Relapse | Distant recurrence/metastasis | Deceased | 472.0 | G12D | Y163C,Y70C,Y31C | No | No |
| DO224705 | Female | PDAC | 59.0 | IIB | G3 | NA | NA | Deceased | 455.0 | G12D | G105C | No | No |
| DO224712 | Male | PDAC | 78.0 | IIB | G3 | Relapse | Distant recurrence/metastasis | Deceased | 239.0 | G12R | R141H,R273H | P94L,P135L | No |
| DO224714 | Male | PDAC | 73.0 | IIB | G3 | Relapse | Distant recurrence/metastasis | Deceased | 437.0 | G12D | G266V,G134V | No | No |
| DO224719 | Male | PDAC | 67.0 | IIA | NA | No evidence of disease | NA | Deceased | 522.0 | No | S127Y,S34Y | No | No |
| DO224724 | Female | PDAC | 63.0 | IIB | G3 | Relapse | Distant recurrence/metastasis | Deceased | 234.0 | G12D | R282W,R150W | H83Y,H32Y,A138V,A97V | No |
| DO224734 | Male | PDAC | 62.0 | IIB | G2 | Relapse | Distant recurrence/metastasis | Deceased | 271.0 | G12D | I122S,I254S | No | No |
| DO224740 | Male | PDAC | 63.0 | IIB | G1 | Relapse | Distant recurrence/metastasis | Deceased | 684.0 | G12V | S346W | P19R,P70R | No |
| DO224745 | Male | PDAC | 55.0 | IIB | G2 | Relapse | Local recurrence and distant metastasis | Deceased | 456.0 | G12D | R248W,R116W,R155W | No | No |
| DO224750 | Female | PDAC | 52.0 | IV | G3 | No evidence of disease | NA | Deceased | 165.0 | No | No | No | E330K,E234K |
| DO224752 | Male | Mucinous adenocarcinoma | 67.0 | IIB | G3 | No evidence of disease | NA | Alive | 257.0 | No | No | No | No |
| DO224758 | Male | PDAC | 58.0 | IIB | G3 | No evidence of disease | NA | Deceased | 65.0 | G12V | No | No | No |
| DO224764 | Male | PDAC | 58.0 | IIB | G2 | No evidence of disease | NA | Alive | 244.0 | G12R | Y126S,Y33S | No | No |
| DO224767 | Male | Adenocarcinoma, NOS | 48.0 | IIB | NA | Relapse | Distant recurrence/metastasis | Deceased | 324.0 | G12D | S127F,S34F | No | No |
| DO224770 | Female | PDAC | 74.0 | IIB | G2 | No evidence of disease | NA | Alive | 200.0 | G12V | No | No | No |
| DO224776 | Male | PDAC | 70.0 | IIB | G2 | Relapse | Distant recurrence/metastasis | Deceased | 201.0 | G12D | R248Q,R155Q,R116Q | No | No |
| DO224779 | Female | PDAC | 69.0 | IIB | G2 | No evidence of disease | NA | Deceased | 228.0 | G12D | R141H,R273H | H83Y,H32Y,A138V,A97V | No |
| DO224782 | Female | PDAC | 59.0 | IIB | NA | Relapse | Distant recurrence/metastasis | Deceased | 389.0 | No | No | No | No |
| DO224784 | Female | PDAC | 72.0 | IIB | G2 | Relapse | Local recurrence and distant metastasis | Deceased | 816.0 | G12D | R273C,R141C | No | No |
| DO227482 | Female | Carcinoma, NOS | NA | NA | NA | NA | NA | Deceased | 347.0 | G12V | R196P,R64P,R103P | No | No |
| DO227485 | Female | Carcinoma, NOS | NA | NA | NA | NA | NA | Alive | NA | G12R | R282G,R150G | No | No |
| DO227531 | Male | Carcinoma, NOS | NA | NA | NA | NA | NA | Deceased | 192.0 | No | Y205S,Y73S,Y112S | No | No |
| DO227544 | Male | Carcinoma, NOS | NA | NA | NA | NA | NA | Deceased | 132.0 | G12D | R175H,R82H,R43H | No | No |
| DO227554 | Female | Carcinoma, NOS | NA | NA | NA | NA | NA | Deceased | 168.0 | G12D | H193Y,H61Y,H100Y | No | No |
| DO227558 | Male | Carcinoma, NOS | NA | NA | NA | NA | NA | Deceased | 216.0 | G12D | No | No | No |
| DO227564 | Male | Carcinoma, NOS | NA | NA | NA | NA | NA | Deceased | 217.0 | G12D | G245S,G152S,G113S | No | V128L |
| DO227570 | Male | Carcinoma, NOS | NA | NA | NA | NA | NA | Deceased | 113.0 | G12V | R282W,R150W | No | No |
| DO227581 | Female | Carcinoma, NOS | NA | NA | NA | NA | NA | Deceased | 177.0 | G12R | No | No | No |
| DO227596 | Male | Carcinoma, NOS | NA | NA | NA | NA | NA | Deceased | 142.0 | G12R | No | No | No |
| DO227604 | Female | Carcinoma, NOS | NA | NA | NA | NA | NA | Deceased | 164.0 | G12D | S83G,S215G,S122G | No | No |
| DO227625 | Male | Carcinoma, NOS | NA | NA | NA | NA | NA | Alive | NA | G12V | No | No | No |
| DO227633 | Male | Carcinoma, NOS | NA | NA | NA | NA | NA | Deceased | 1074.0 | G12V | G245S,G152S,G113S | No | No |
| DO227636 | Female | Carcinoma, NOS | NA | NA | NA | NA | NA | Alive | NA | G12R | No | No | No |
| DO227648 | Female | Carcinoma, NOS | NA | NA | NA | NA | NA | Deceased | 311.0 | G12R | K132R,K39R | No | No |
| DO227652 | Male | Carcinoma, NOS | NA | NA | NA | NA | NA | Alive | NA | G12D | No | No | No |
| DO227661 | Male | Carcinoma, NOS | NA | NA | NA | NA | NA | Alive | NA | No | No | No | No |
| DO227671 | Female | Carcinoma, NOS | NA | NA | NA | NA | NA | Deceased | 673.0 | G12D | G266R,G134R | No | No |
| DO227684 | Female | Carcinoma, NOS | NA | NA | NA | NA | NA | Deceased | 745.0 | G12V | R175H,R82H,R43H | No | No |
| DO227687 | Male | Carcinoma, NOS | NA | NA | NA | NA | NA | Deceased | 823.0 | G12D | A159V,A27V,A66V | No | No |
| DO227695 | Female | Carcinoma, NOS | NA | NA | NA | NA | NA | Deceased | 531.0 | G12R | A45V,A138V,A6V,A131V | D108G,D57G | No |
| DO227704 | Male | Carcinoma, NOS | NA | NA | NA | NA | NA | Deceased | 681.0 | G12V | No | No | R361S,R265S |
| DO227721 | Male | Carcinoma, NOS | NA | NA | NA | NA | NA | Alive | NA | G12V | K132N,K39N | No | No |
| DO227727 | Male | Carcinoma, NOS | NA | NA | NA | NA | NA | Alive | NA | G12R | R248Q,R155Q,R116Q | G166R,G125R | No |
| DO227736 | Male | Carcinoma, NOS | NA | NA | NA | NA | NA | Deceased | 158.0 | G12V | R273G,R141G | No | No |
| DO227742 | Male | Carcinoma, NOS | NA | NA | NA | NA | NA | Deceased | 482.0 | G12V | R175H,R82H,R43H | No | No |
| DO230463 | Male | Carcinoma, NOS | NA | NA | NA | NA | NA | Deceased | 574.0 | G12R | P278S,P146S | No | No |
| DO230464 | Female | Carcinoma, NOS | NA | NA | NA | NA | NA | Deceased | 170.0 | G12D | No | No | No |
| DO230465 | Male | Carcinoma, NOS | NA | NA | NA | NA | NA | Deceased | 0.0 | No | No | No | No |
| DO231247 | Male | Carcinoma, NOS | NA | NA | NA | NA | NA | Alive | NA | G12R | R282W,R150W | No | No |
| DO231251 | Female | Carcinoma, NOS | NA | NA | NA | NA | NA | Alive | NA | G12V | No | No | No |
| DO231256 | Male | Carcinoma, NOS | NA | NA | NA | NA | NA | Alive | NA | G12D | S127Y,S34Y | No | No |
| DO231259 | NA | Carcinoma, NOS | NA | NA | NA | NA | NA | NA | NA | No | No | No | No |
| DO231264 | Female | Carcinoma, NOS | NA | NA | NA | NA | NA | Alive | NA | G12V | D281G,D149G | D57V,D108V | No |
| DO231272 | NA | Carcinoma, NOS | NA | NA | NA | NA | NA | NA | NA | No | No | No | No |
| DO231278 | NA | Carcinoma, NOS | NA | NA | NA | NA | NA | NA | NA | G12V | R273C,R141C | No | No |
| DO231284 | Female | Carcinoma, NOS | NA | NA | NA | NA | NA | Alive | NA | Q61H | No | No | No |
| DO35081 | NA | Carcinoma, NOS | NA | NA | NA | NA | NA | NA | NA | No | No | No | No |
| DO35082 | Male | PDAC | 68.0 | IIB | G1 | Complete remission | NA | Deceased | 371.0 | Q61H | No | No | No |
| DO35083 | Female | PDAC | 68.0 | IIB | G2 | Complete remission | NA | Alive | 1264.0 | G12V | No | P94L,P135L,P72L,P113L | No |
| DO35084 | Male | Carcinoma, NOS | 74.0 | T3N1MX | NA | Complete remission | NA | Deceased | 131.0 | G12D | No | No | No |
| DO35085 | Male | PDAC | 59.0 | IIA | G2 | Relapse | Local recurrence and distant metastasis | Deceased | 224.0 | G12D | No | No | No |
| DO35086 | Female | Carcinoma, NOS | 66.0 | T3N1MX | NA | NA | NA | Deceased | 590.0 | G12V | R248L,R116L,R155L | No | No |
| DO35088 | Female | Carcinoma, NOS | 71.0 | T3N1MX | G2 | Complete remission | NA | Alive | 35.0 | Q61H | No | No | No |
| DO35090 | Male | Carcinoma, NOS | 72.0 | NA | G2 | Partial remission | NA | Alive | 1287.0 | G12V | No | No | No |
| DO35092 | NA | Carcinoma, NOS | 63.0 | IV | G3 | Relapse | Distant recurrence/metastasis | NA | 1441.0 | No | No | No | No |
| DO35094 | Female | Carcinoma, NOS | 68.0 | IIB | G2 | Complete remission | NA | NA | NA | G12V | No | No | S72I |
| DO35096 | NA | Carcinoma, NOS | NA | TXNXMX | NA | NA | NA | NA | NA | No | No | No | No |
| DO35098 | Male | PDAC | 63.0 | IIB | G2 | Progression | Distant recurrence/metastasis | Deceased | 1499.0 | G12R | No | No | No |
| DO35100 | Male | Carcinoma, NOS | 78.0 | IIA | G3 | NA | NA | Deceased | 233.0 | G12D | S127F,S34F | No | No |
| DO35102 | Male | Carcinoma, NOS | 44.0 | TXNXMX | G3 | Relapse | Local recurrence | Deceased | 572.0 | G12D,G12R | Y205C,Y112C,Y73C | No | No |
| DO35104 | Male | Carcinoma, NOS | 88.0 | NA | NA | NA | NA | Deceased | 1091.0 | G12V | M105I,M237I,M144I | No | No |
| DO35106 | Male | Carcinoma, NOS | 66.0 | T3N1MX | NA | Complete remission | NA | NA | 14.0 | G12D | No | No | No |
| DO35110 | Female | Carcinoma, NOS | 88.0 | T3N1MX | NA | NA | NA | NA | NA | G12D | No | No | No |
| DO35112 | Male | Carcinoma, NOS | 62.0 | IIB | G3 | NA | NA | Deceased | 1189.0 | G12D | V172F,V40F,V79F | No | No |
| DO35114 | Female | PDAC | 65.0 | NA | G3 | NA | NA | Deceased | 1738.0 | G12D | No | No | No |
| DO35116 | Female | PDAC | 76.0 | IV | G2 | Complete remission | NA | Alive | 1646.0 | G12V | No | No | No |
| DO35118 | Female | PDAC | 79.0 | IIB | G4 | NA | NA | Deceased | 285.0 | G12D | No | No | No |
| DO35120 | Male | Carcinoma, NOS | 80.0 | T3N1MX | G3 | Relapse | Distant recurrence/metastasis | Alive | 641.0 | G12V | No | No | No |
| DO35122 | Female | PDAC | 53.0 | III | G1 | Complete remission | NA | Alive | 2356.0 | G12V | No | No | No |
| DO35124 | NA | Carcinoma, NOS | NA | TXNXMX | NA | NA | NA | NA | NA | No | No | No | No |
| DO35126 | Female | PDAC | 55.0 | IIB | G2 | Complete remission | NA | Deceased | 280.0 | G12V | No | No | L57S |
| DO35128 | Female | PDAC | 83.0 | IB | G2 | Progression | Distant recurrence/metastasis | Deceased | 361.0 | G12V | No | No | No |
| DO35130 | NA | Carcinoma, NOS | NA | T3N1MX | NA | NA | NA | NA | NA | No | No | No | No |
| DO35132 | Male | PDAC | 69.0 | IIA | G2 | NA | NA | Deceased | 90.0 | G12D | V85G,V217G,V124G | No | No |
| DO35134 | Male | Carcinoma, NOS | 46.0 | T3N1MX | G1 | Complete remission | NA | Alive | 28.0 | G12D | No | No | No |
| DO35136 | Female | PDAC | 61.0 | IB | G3 | Complete remission | NA | Alive | 1347.0 | Q61R | No | No | E330K,E234K,D255H,D351H,G352E,G256E |
| DO35138 | Female | Adenosquamous carcinoma | 69.0 | IIB | G3 | Relapse | Distant recurrence/metastasis | Deceased | 247.0 | No | No | No | No |
| DO35140 | Female | PDAC | 54.0 | IIB | G2 | Progression | Distant recurrence/metastasis | Deceased | 1262.0 | G12V | R141H,R273H | No | No |
| DO35142 | NA | Carcinoma, NOS | NA | NA | NA | NA | NA | NA | NA | No | No | No | No |
| DO35144 | Male | PDAC | 69.0 | IIB | G2 | Relapse | Local recurrence | Deceased | 311.0 | G12V | C83S,C176S,C44S | No | No |
| DO35146 | NA | Carcinoma, NOS | 61.0 | T3N1MX | G3 | Relapse | Local recurrence | NA | 10.0 | G12C | L145Q,L52Q,L13Q,L138Q | No | No |
| DO35148 | Female | PDAC | 77.0 | IB | G2 | Relapse | Distant recurrence/metastasis | Deceased | 1013.0 | G12D | R282W,R150W | No | No |
| DO35150 | Male | Carcinoma, NOS | 48.0 | IV | NA | Relapse | Distant recurrence/metastasis | Alive | 1304.0 | G12D | No | No | R361H,R265H |
| DO35152 | Male | PDAC | 55.0 | IIB | G3 | Progression | Distant recurrence/metastasis | Deceased | 330.0 | G12D | No | No | No |
| DO35154 | Female | Carcinoma, NOS | 72.0 | T2N1MX | NA | Complete remission | NA | Alive | 818.0 | G12V | No | No | No |
| DO35156 | Male | Carcinoma, NOS | 76.0 | T3N1MX | G4 | Relapse | Distant recurrence/metastasis | Deceased | 383.0 | G12D | No | No | No |
| DO35158 | Female | Carcinoma, NOS | 76.0 | TXNXMX | G2 | Relapse | NA | Deceased | 761.0 | G12V,G12D | No | No | No |
| DO35160 | Female | Carcinoma, NOS | 48.0 | TXNXMX | NA | NA | NA | Deceased | 209.0 | No | No | No | No |
| DO35162 | Female | Carcinoma, NOS | 75.0 | IIA | G4 | NA | NA | Deceased | 286.0 | G12V | No | No | No |
| DO35164 | NA | Carcinoma, NOS | NA | T3N1MX | NA | NA | NA | NA | NA | No | No | No | No |
| DO35166 | Male | Carcinoma, NOS | 66.0 | TXNXMX | G3 | Partial remission | Distant recurrence/metastasis | Alive | 181.0 | G12D | C83F,C176F,C44F | No | No |
| DO35168 | NA | Carcinoma, NOS | NA | NA | NA | NA | NA | NA | NA | No | No | No | No |
| DO35170 | NA | Carcinoma, NOS | NA | NA | NA | NA | NA | NA | NA | No | No | No | No |
| DO35172 | NA | Carcinoma, NOS | 65.0 | T3N1MX | G3 | Relapse | Local recurrence | NA | 411.0 | G12D | No | A17G | No |
| DO35174 | Male | Carcinoma, NOS | 84.0 | T3N0MX | G2 | Complete remission | NA | NA | 720.0 | G12D | A161T,A29T,A68T | No | No |
| DO35176 | Male | Carcinoma, NOS | 72.0 | T3N0MX | G2 | Complete remission | NA | Alive | 182.0 | G12D | R175H,R82H,R43H | No | No |
| DO35178 | Male | Carcinoma, NOS | 59.0 | TXNXMX | NA | NA | NA | Deceased | 361.0 | G12D | No | No | No |
| DO35180 | Female | Carcinoma, NOS | 77.0 | IIB | G3 | NA | NA | Deceased | 232.0 | G12V | M105I,M237I,M144I | No | No |
| DO35182 | NA | Carcinoma, NOS | NA | TXNXMX | NA | NA | NA | NA | NA | No | No | No | No |
| DO35184 | Male | PDAC | 75.0 | IB/IIB | G1 | Complete remission | NA | Deceased | 102.0 | G12D | No | L130Q,L79Q | No |
| DO35186 | Female | PDAC | 76.0 | IIA | G3 | Relapse | NA | Deceased | NA | G12V | No | No | No |
| DO35188 | Female | Carcinoma, NOS | 58.0 | T3N1MX | G2 | Complete remission | NA | Alive | 111.0 | G12V | No | No | No |
| DO35190 | NA | Carcinoma, NOS | NA | TXNXMX | NA | NA | NA | NA | NA | No | No | No | No |
| DO35192 | Male | Carcinoma, NOS | 34.0 | T3N1MX | NA | Relapse | Distant recurrence/metastasis | Deceased | 410.0 | G12V | D259V,D127V | No | No |
| DO35194 | NA | Carcinoma, NOS | NA | NA | NA | NA | NA | NA | NA | No | No | No | No |
| DO35196 | Male | Carcinoma, NOS | 71.0 | III | G4 | NA | NA | Deceased | NA | G12V | R158H,R65H,R26H | No | No |
| DO35198 | Female | PDAC | 70.0 | IIB | G3 | Complete remission | NA | Deceased | 463.0 | G12V | No | No | No |
| DO35200 | Female | PDAC | 62.0 | IIB | G3 | Complete remission | NA | Alive | 320.0 | G12V | R282W,R150W | No | No |
| DO35202 | NA | Carcinoma, NOS | NA | NA | NA | NA | NA | NA | NA | No | No | No | No |
| DO35204 | Male | PDAC | 61.0 | IIB | G3 | Relapse | NA | Deceased | NA | G12V | No | No | L437P,L533P |
| DO35206 | Female | Carcinoma, NOS | 82.0 | T3N1MX | G2 | Complete remission | NA | Alive | 772.0 | G12V | R175H,R82H,R43H | No | No |
| DO35208 | Female | Carcinoma, NOS | 43.0 | TXNXMX | NA | NA | NA | Deceased | 253.0 | G12V | No | No | No |
| DO35210 | Male | Carcinoma, NOS | 75.0 | IB | G1 | NA | NA | Deceased | 886.0 | G12D,G12R | D281E,D149E,R248Q,R155Q,R116Q | No | No |
| DO35212 | Male | Carcinoma, NOS | 43.0 | TXNXMX | NA | NA | NA | Deceased | NA | G12D | R248Q,R155Q,R116Q | No | No |
| DO35214 | NA | Carcinoma, NOS | NA | TXNXMX | NA | NA | NA | NA | NA | No | No | No | No |
| DO35216 | Female | Adenocarcinoma, NOS | 63.0 | IIB | G3 | Relapse | Distant recurrence/metastasis | Deceased | 857.0 | G12V | No | No | No |
| DO35218 | Male | Carcinoma, NOS | 56.0 | T3N1MX | G2 | Partial remission | Distant recurrence/metastasis | Alive | 595.0 | G12D | R175H,R82H,R43H | No | No |
| DO35220 | NA | Carcinoma, NOS | NA | TXNXMX | NA | NA | NA | NA | NA | No | No | No | No |
| DO35222 | Male | PDAC | 53.0 | IIB | G2 | Progression | Local recurrence | Deceased | 1234.0 | Q61H | No | No | No |
| DO35224 | Female | Carcinoma, NOS | 57.0 | TXNXMX | NA | NA | NA | Deceased | 361.0 | No | No | No | No |
| DO35226 | Female | PDAC | 50.0 | IB | G3 | Relapse | Local recurrence | Deceased | 627.0 | G12R | No | No | No |
| DO35228 | Male | PDAC | 68.0 | IIA | G2 | Complete remission | NA | Alive | 1623.0 | G12D | G262V,G130V | No | No |
| DO35230 | Female | PDAC | 52.0 | IIB | G2 | Complete remission | NA | Deceased | 263.0 | G12D | No | No | No |
| DO35232 | NA | Carcinoma, NOS | NA | NA | NA | NA | NA | NA | NA | No | No | No | No |
| DO35234 | Female | Carcinoma, NOS | 84.0 | T3N0MX | G3 | Complete remission | Distant recurrence/metastasis | Alive | 91.0 | G12D | No | H83Y,H32Y,A138V,A97V | No |
| DO35236 | Female | PDAC | 58.0 | IIB | G2 | Progression | Local recurrence | Alive | 1343.0 | Q61H | No | No | No |
| DO35239 | NA | Carcinoma, NOS | NA | NA | NA | NA | NA | NA | NA | G12D | R280G,R148G | No | No |
| DO35242 | Male | Carcinoma, NOS | 51.0 | NA | NA | Relapse | Distant recurrence/metastasis | Deceased | 201.0 | No | No | No | No |
| DO35245 | Male | Carcinoma, NOS | 76.0 | IIB | G2 | NA | NA | Deceased | NA | G12D | No | No | No |
| DO35248 | Male | Carcinoma, NOS | 74.0 | IB | G1 | NA | NA | Alive | 423.0 | No | No | No | No |
| DO35251 | NA | Carcinoma, NOS | NA | NA | NA | NA | NA | NA | NA | No | No | No | No |
| DO35254 | Male | Carcinoma, NOS | 47.0 | IV | G4 | NA | NA | Deceased | 1431.0 | G12V | No | No | No |
| DO35258 | Male | PDAC | 82.0 | IIB | G2 | Progression | Local recurrence | Deceased | 362.0 | G12D | G266E,G134E | No | No |
| DO35262 | NA | Carcinoma, NOS | NA | NA | NA | NA | NA | NA | NA | No | No | No | No |
| DO35266 | Male | Carcinoma, NOS | 79.0 | T3N1MX | NA | Complete remission | NA | Alive | 24.0 | No | No | No | No |
| DO35270 | Male | Carcinoma, NOS | 73.0 | T3N1MX | G2 | Relapse | Distant recurrence/metastasis | Deceased | 217.0 | G12D | C275G,C143G | No | No |
| DO35275 | Male | Carcinoma, NOS | 64.0 | T3N1MX | NA | Progression | NA | Deceased | 433.0 | G12R | C141Y,C9Y,C48Y,C134Y | No | No |
| DO35280 | NA | Carcinoma, NOS | NA | NA | NA | NA | NA | NA | NA | No | No | No | No |
| DO35285 | NA | Carcinoma, NOS | 88.0 | T3N1MX | G3 | Relapse | NA | NA | 7.0 | No | No | No | No |
| DO35290 | Male | PDAC | 65.0 | IIB | G2 | Relapse | Local recurrence and distant metastasis | Deceased | 673.0 | G12R | L145Q,L52Q,L13Q,L138Q | No | No |
| DO35295 | NA | Carcinoma, NOS | NA | NA | NA | NA | NA | NA | NA | No | No | No | No |
| DO35300 | NA | Carcinoma, NOS | NA | NA | NA | NA | NA | NA | NA | No | No | No | No |
| DO35305 | Male | PDAC | 70.0 | IIB | G2 | Relapse | Local recurrence | Deceased | 746.0 | G12V | No | No | No |
| DO35310 | NA | Carcinoma, NOS | NA | NA | NA | NA | NA | NA | NA | No | No | No | No |
| DO35315 | Male | Carcinoma, NOS | 56.0 | T3N1MX | G2 | Complete remission | NA | Alive | 21.0 | G12D | R213L,R120L,R81L | No | No |
| DO35320 | NA | Carcinoma, NOS | NA | NA | NA | NA | NA | NA | NA | No | No | No | No |
| DO35325 | Male | Carcinoma, NOS | 66.0 | IIB | G2 | Relapse | Distant recurrence/metastasis | Deceased | 618.0 | G12V | No | No | No |
| DO35330 | Male | PDAC | 63.0 | IIB | G2 | Relapse | Local recurrence and distant metastasis | Deceased | 1359.0 | No | No | No | No |
| DO35335 | NA | Carcinoma, NOS | NA | NA | NA | NA | NA | NA | NA | No | No | No | No |
| DO35340 | NA | Carcinoma, NOS | NA | NA | NA | NA | NA | NA | NA | No | No | No | No |
| DO35345 | Male | Carcinoma, NOS | 75.0 | TXNXMX | NA | NA | NA | Deceased | 235.0 | G12V | No | No | No |
| DO35350 | Male | PDAC | 76.0 | IIB | G2 | Relapse | Distant recurrence/metastasis | Deceased | 146.0 | G12R | No | No | No |
| DO35355 | Male | Carcinoma, NOS | 75.0 | IB | G3 | NA | NA | Alive | NA | No | No | No | No |
| DO35360 | Male | PDAC | 65.0 | IIA | G2 | Complete remission | NA | Alive | 2174.0 | G12R | No | No | No |
| DO35365 | Male | PDAC | 61.0 | IIB | G1 | Partial remission | NA | Alive | 889.0 | G12D | R141H,R273H | R36W,R87W,P101L,P142L | No |
| DO35370 | NA | Carcinoma, NOS | 48.0 | T3N0MX | G2 | Partial remission | Distant recurrence/metastasis | NA | 421.0 | No | No | No | No |
| DO35376 | Female | PDAC | 42.0 | IIB | G1 | Progression | Distant recurrence/metastasis | Deceased | 877.0 | G12D | V272M,V140M | No | No |
| DO35382 | Male | Carcinoma, NOS | 63.0 | IIB | G2 | Relapse | Distant recurrence/metastasis | Deceased | 166.0 | No | No | No | No |
| DO35388 | Male | Carcinoma, NOS | 67.0 | IIB | G2 | Relapse | Distant recurrence/metastasis | Deceased | 1001.0 | G12V | C242F,C149F,C110F | No | No |
| DO35394 | Male | Carcinoma, NOS | 62.0 | T3N1MX | G3 | Partial remission | Distant recurrence/metastasis | Alive | 523.0 | G12D | P250L,P118L | No | No |
| DO35400 | NA | Carcinoma, NOS | NA | NA | NA | NA | NA | NA | NA | No | No | No | No |
| DO35406 | Male | PDAC | 54.0 | III | G2 | Complete remission | NA | Deceased | 160.0 | G12D | No | No | No |
| DO35412 | NA | Carcinoma, NOS | NA | NA | NA | NA | NA | NA | NA | No | No | No | No |
| DO35418 | Male | Carcinoma, NOS | 75.0 | T3N1MX | G2 | Partial remission | Distant recurrence/metastasis | Alive | 390.0 | No | No | No | No |
| DO35424 | Male | PDAC | 74.0 | IIB | G2 | Progression | Distant recurrence/metastasis | Deceased | 575.0 | G12V | No | No | No |
| DO35430 | NA | Carcinoma, NOS | NA | NA | NA | NA | NA | NA | NA | No | No | No | No |
| DO35436 | NA | Carcinoma, NOS | NA | NA | NA | NA | NA | NA | NA | No | No | No | No |
| DO35442 | Female | Carcinoma, NOS | 53.0 | IIB | G4 | Complete remission | NA | Alive | 1331.0 | No | No | No | No |
| DO35448 | NA | Carcinoma, NOS | NA | NA | NA | NA | NA | NA | NA | No | No | No | No |
| DO35454 | Female | PDAC | 74.0 | IIA | G2 | Relapse | Distant recurrence/metastasis | Deceased | 315.0 | G12R | No | No | No |
| DO35460 | NA | Carcinoma, NOS | 76.0 | T3N0MX | G2 | Complete remission | NA | NA | 339.0 | G12D | P278L,P146L | No | No |
| DO35466 | NA | Carcinoma, NOS | NA | NA | NA | NA | NA | NA | NA | No | No | No | No |
| DO35472 | NA | Carcinoma, NOS | NA | NA | NA | NA | NA | NA | NA | No | No | No | No |
| DO35478 | Female | Carcinoma, NOS | 50.0 | TXNXMX | NA | NA | NA | Alive | 938.0 | G12D | No | No | No |
| DO35484 | Male | Carcinoma, NOS | 44.0 | IIB | G4 | Progression | NA | Alive | NA | G12D | P151S,P58S,P19S | No | No |
| DO35490 | NA | Carcinoma, NOS | NA | NA | NA | NA | NA | NA | NA | No | No | No | No |
| DO35496 | Female | PDAC | 63.0 | IIB | G2 | Progression | Distant recurrence/metastasis | Deceased | 1072.0 | Q61H | V80L,V173L,V41L | No | C127F |
| DO35502 | Male | Carcinoma, NOS | 70.0 | TXNXMX | NA | NA | NA | Deceased | 562.0 | G12D | L257P,L125P | No | No |
| DO35508 | Male | Carcinoma, NOS | 59.0 | IIB | G2 | Relapse | NA | Deceased | 255.0 | No | No | No | No |
| DO35514 | Female | Carcinoma, NOS | 72.0 | T3N0MX | G2 | Partial remission | Distant recurrence/metastasis | Alive | 787.0 | No | No | No | No |
| DO35520 | NA | Carcinoma, NOS | NA | NA | NA | NA | NA | NA | NA | No | No | No | No |
| DO35526 | NA | Carcinoma, NOS | NA | NA | NA | NA | NA | NA | NA | No | No | No | No |
| DO35532 | NA | Carcinoma, NOS | NA | NA | NA | NA | NA | NA | NA | No | No | No | No |
| DO49418 | Male | PDAC | 58.0 | IB | G1 | Progression | Distant recurrence/metastasis | Deceased | 1448.0 | No | R181H,R88H,R49H | No | No |
| DO49419 | Male | PDAC | 50.0 | IIB | G2 | Progression | Distant recurrence/metastasis | Deceased | 681.0 | G12R | No | No | No |
| DO49420 | Male | PDAC | 64.0 | IIB | G2 | Relapse | Distant recurrence/metastasis | Alive | 1164.0 | G12D | No | No | No |
| DO49421 | Female | IPMN | 74.0 | III | G3 | Relapse | Distant recurrence/metastasis | Deceased | 385.0 | G12R | No | L79P,L130P | No |
| DO49422 | Male | Adenocarcinoma, NOS | 74.0 | IIB | G4 | NA | NA | Deceased | 475.0 | G12D | C242S,C110S,C149S | No | No |
| DO49424 | Male | PDAC | 84.0 | IIB | G2 | Relapse | Local recurrence and distant metastasis | Deceased | 341.0 | G12V | R141H,R273H | No | G386V,G290V |
| DO49427 | Female | PDAC | 69.0 | IIA | G3 | Complete remission | NA | Alive | 1483.0 | G12R | No | No | No |
| DO49430 | Male | PDAC | 81.0 | IIA | G3 | Relapse | NA | Deceased | 821.0 | No | No | No | No |
| DO49433 | Male | PDAC | 48.0 | IIB | G3 | Relapse | Distant recurrence/metastasis | Deceased | 456.0 | G12D | No | No | No |
| DO49436 | Female | PDAC | 69.0 | IIB | G3 | Relapse | Distant recurrence/metastasis | Deceased | 663.0 | G12R | G245S,G152S,G113S | No | No |
| DO49439 | Female | PDAC | 79.0 | IIB | G3 | Progression | Local recurrence | Deceased | 608.0 | G12D | No | No | No |
| DO49442 | Male | PDAC | 57.0 | IIB | G1 | Complete remission | NA | Deceased | 995.0 | No | No | No | No |
| DO49445 | Female | PDAC | 60.0 | IIB | G1 | Complete remission | NA | Alive | 1030.0 | G12V | No | No | No |
| DO49448 | Male | PDAC | 68.0 | IIB | G2 | Progression | Distant recurrence/metastasis | Deceased | 951.0 | G12R | No | No | K332N,K428N,K17N |
| DO49451 | Male | PDAC | 66.0 | IIB | G3 | Progression | Local recurrence | Deceased | 918.0 | G12V | R337C | No | No |
| DO49454 | Female | PDAC | 74.0 | IIB | G2 | Progression | Distant recurrence/metastasis | Deceased | 294.0 | G12R | No | No | G352V,G256V |
| DO49457 | Female | PDAC | 53.0 | IIA | G2 | Complete remission | NA | Alive | 904.0 | G12D | No | H83Y,H32Y,A138V,A97V | No |
| DO49460 | Female | PDAC | 64.0 | IIB | G2 | Progression | Distant recurrence/metastasis | Deceased | 767.0 | G12R | No | No | No |
| DO49463 | Female | PDAC | 78.0 | IIB | G3 | Relapse | Distant recurrence/metastasis | Deceased | 730.0 | Q61H | G245S,G152S,G113S | No | No |
| DO49466 | Male | PDAC | 79.0 | IIB | G3 | Relapse | Local recurrence and distant metastasis | Deceased | 312.0 | G12R | No | No | No |
| DO49469 | Male | PDAC | 78.0 | IIB | G2 | Progression | Local recurrence | Deceased | 573.0 | G12V | G152D,G245D,G113D | No | No |
| DO49472 | Male | PDAC | 66.0 | IIB | G2 | Progression | Distant recurrence/metastasis | Deceased | 578.0 | G12D | No | G60V,G111V | G352V,G256V |
| DO49475 | Male | PDAC | 74.0 | IIB | G2 | Relapse | Distant recurrence/metastasis | Deceased | 628.0 | G12D | No | No | No |
| DO49478 | Male | PDAC | 81.0 | IIA | G1 | Complete remission | NA | Alive | 653.0 | G13A,G13R | R141H,R273H | No | No |
| DO49481 | Male | PDAC | 64.0 | IIB | G2 | Relapse | NA | Deceased | 383.0 | G12V | No | No | No |
| DO49484 | Male | PDAC | 67.0 | IIB | G2 | Complete remission | NA | Alive | 287.0 | G12R | No | No | No |
| DO51464 | Male | PDAC | 72.0 | IIA | G1 | No evidence of disease | NA | Deceased | 144.0 | G12V | R175H,R82H,R43H | No | No |
| DO51465 | Female | PDAC | 74.0 | IIB | G1 | Progression | Distant recurrence/metastasis | Deceased | 1226.0 | G12D | No | No | H92Y |
| DO51466 | Male | PDAC | 69.0 | IIB | G2 | Progression | Local recurrence and distant metastasis | Deceased | 351.0 | G12V | C128W,C135W,C42W,C3W | No | No |
| DO51467 | Male | PDAC | 67.0 | III | G3 | Relapse | Local recurrence | Deceased | 471.0 | G12V | S241F,S148F,S109F | No | No |
| DO51468 | Male | Mucinous adenocarcinoma | 69.0 | III | G3 | Progression | Distant recurrence/metastasis | Deceased | 1083.0 | G12V | No | No | No |
| DO51469 | Female | PDAC | 80.0 | IIB | G3 | Relapse | Distant recurrence/metastasis | Deceased | 314.0 | G12D | Y205H,Y73H,Y112H | No | No |
| DO51470 | Female | PDAC | 51.0 | IIB | G2 | Relapse | Local recurrence and distant metastasis | Deceased | 444.0 | G12V | R248W,R116W,R155W | No | No |
| DO51472 | Female | PDAC | 65.0 | IIB | G2 | Progression | Distant recurrence/metastasis | Deceased | 590.0 | G12V | C176W,C44W,C83W | No | No |
| DO51473 | Female | Adenocarcinoma, NOS | 76.0 | IIB | G3 | Complete remission | NA | Deceased | 4289.0 | G12V | No | No | No |
| DO51474 | Male | PDAC | 63.0 | IB | G3 | Complete remission | NA | Alive | 1633.0 | G12R | No | No | No |
| DO51475 | Female | PDAC | 58.0 | IIB | G1 | Progression | Distant recurrence/metastasis | Deceased | 1824.0 | G12V | R248W,R116W,R155W | No | No |
| DO51476 | Female | Mucinous adenocarcinoma | 45.0 | IIB | G3 | Relapse | Local recurrence | Deceased | 1576.0 | No | R175H,R82H,R43H | No | No |
| DO51477 | Male | Carcinoma, NOS | 56.0 | IIB | G3 | Complete remission | NA | Alive | 504.0 | G12V | No | No | No |
| DO51478 | Male | PDAC | 69.0 | IIB | G2 | Relapse | Distant recurrence/metastasis | Deceased | 311.0 | G12D | No | No | No |
| DO51479 | Male | PDAC | 70.0 | NA | G3 | NA | NA | Alive | 1691.0 | No | R273C,R141C | No | No |
| DO51480 | Female | Adenocarcinoma, NOS | 41.0 | IIB | G3 | Relapse | Distant recurrence/metastasis | Deceased | 228.0 | G12V | R175H,R82H,R43H | No | L437P,L533P |
| DO51481 | Female | PDAC | 59.0 | IIA | G3 | Relapse | Distant recurrence/metastasis | Deceased | 587.0 | G12V | No | No | No |
| DO51482 | Male | PDAC | 73.0 | IIA | G2 | Relapse | NA | Deceased | 379.0 | G12D | No | No | No |
| DO51483 | Female | Adenocarcinoma, NOS | 67.0 | IIB | G3 | Relapse | Distant recurrence/metastasis | Deceased | 107.0 | G12D | Y220C,Y127C,Y88C | No | No |
| DO51484 | Female | PDAC | 63.0 | IA | G1 | Relapse | Local recurrence and distant metastasis | Deceased | 1460.0 | G12V | D281N,D149N | R139Q,R98Q,D33N,D84N | No |
| DO51485 | Female | PDAC | 74.0 | IIB | G3 | Relapse | Local recurrence | Deceased | 1879.0 | G12D | R273C,R141C | No | No |
| DO51486 | Female | Carcinoma, NOS | 56.0 | IIB | G1 | Complete remission | NA | Alive | 518.0 | G12V | V123L,V216L,V84L | No | No |
| DO51487 | Female | Adenocarcinoma, NOS | 69.0 | IIB | G4 | Complete remission | NA | Alive | 2555.0 | No | No | No | No |
| DO51488 | Female | Carcinoma, NOS | 55.0 | IIB | G3 | Complete remission | NA | Alive | 579.0 | G12V | No | No | No |
| DO51489 | Female | PDAC | 60.0 | IIA | G2 | Complete remission | NA | Alive | 1253.0 | No | R248Q,R155Q,R116Q | No | No |
| DO51490 | Female | PDAC | 78.0 | IIB | G2 | Relapse | Distant recurrence/metastasis | Deceased | 232.0 | G12D | No | H83Y,H32Y,A138V,A97V | No |
| DO51491 | Male | PDAC | 83.0 | IIA | G2 | Complete remission | NA | Alive | 539.0 | No | R158P,R26P,R65P | W110R,W59R,L124P,L165P | No |
| DO51492 | Female | PDAC | 57.0 | IIB | G2 | Relapse | Distant recurrence/metastasis | Deceased | 282.0 | G12V | No | No | No |
| DO51493 | Female | Adenocarcinoma, NOS | 52.0 | IIB | G3 | Relapse | Distant recurrence/metastasis | Deceased | 512.0 | G12V | No | No | No |
| DO51494 | Male | Adenocarcinoma, NOS | 59.0 | IIB | G3 | Relapse | Distant recurrence/metastasis | Deceased | 631.0 | G12V | No | No | No |
| DO51495 | Male | PDAC | 66.0 | IIB | G3 | Relapse | Local recurrence | Deceased | 729.0 | G12V | R175H,R82H,R43H | L16P | No |
| DO51496 | Female | PDAC | 70.0 | IIB | G3 | Complete remission | NA | Deceased | 197.0 | G12R | No | No | No |
| DO51497 | Female | PDAC | 70.0 | IIB | G2 | Relapse | Local recurrence | Deceased | 313.0 | G12R | R175H,R82H,R43H | No | No |
| DO51498 | Female | PDAC | 40.0 | IIB | NA | Relapse | Local recurrence | Deceased | 294.0 | G12D | H179R,H47R,H86R | No | No |
| DO51499 | Male | PDAC | 64.0 | IIB | G2 | Progression | Distant recurrence/metastasis | Deceased | 222.0 | G12R | No | No | No |
| DO51500 | Female | Carcinoma, NOS | 69.0 | NA | G2 | Complete remission | NA | Alive | 482.0 | G13D | No | No | No |
| DO51501 | Male | PDAC | 53.0 | IIB | G3 | Progression | Distant recurrence/metastasis | Deceased | 294.0 | G12D | No | No | L43F |
| DO51502 | Male | Adenocarcinoma, NOS | 47.0 | III | G3 | Relapse | Distant recurrence/metastasis | Deceased | 310.0 | No | No | R139Q,R98Q,D33N,D84N | No |
| DO51503 | Female | PDAC | 46.0 | IIB | G3 | Relapse | Distant recurrence/metastasis | Deceased | 837.0 | No | N268I,N136I | No | No |
| DO51504 | Female | PDAC | 67.0 | IIB | G2 | Relapse | Distant recurrence/metastasis | Deceased | 494.0 | G12R | No | No | No |
| DO51505 | Female | PDAC | 64.0 | IIB | G2 | Relapse | Local recurrence and distant metastasis | Deceased | 351.0 | Q61L | R141H,R273H | No | No |
| DO51506 | Female | PDAC | 62.0 | IIA | G2 | Progression | Local recurrence | Deceased | 1068.0 | G12R | V272M,V140M | No | No |
| DO51507 | Male | PDAC | 73.0 | IIB | G3 | Complete remission | NA | Deceased | 2571.0 | Q61R | V197M,V65M,V104M | No | No |
| DO51508 | Female | Carcinoma, NOS | 83.0 | IIB | G3 | Complete remission | NA | NA | 150.0 | No | No | No | No |
| DO51509 | Male | Adenocarcinoma, NOS | 66.0 | IIB | G3 | Relapse | NA | Deceased | 515.0 | G12V | R248W,R116W,R155W | No | G365D,G269D |
| DO51510 | Male | PDAC | 43.0 | IIB | G1 | Complete remission | NA | Deceased | 287.0 | G12A | No | No | No |
| DO51511 | Female | PDAC | 67.0 | IIB | G2 | Complete remission | NA | Deceased | 592.0 | G12R | Y220C,Y127C,Y88C | No | No |
| DO51512 | Male | PDAC | 42.0 | IIB | G3 | Progression | Local recurrence and distant metastasis | Deceased | 184.0 | G12D | F134L,F2L,F127L,F41L | No | No |
| DO51513 | Female | PDAC | 85.0 | IIB | G1 | Complete remission | NA | Alive | 400.0 | G12D | No | No | No |
| DO51514 | Male | PDAC | 61.0 | IIB | G1 | Progression | Distant recurrence/metastasis | Deceased | 1191.0 | G12V | No | No | No |
| DO51515 | Male | Mucinous adenocarcinoma | 73.0 | IIA | G1 | Complete remission | NA | Alive | 1799.0 | G12V | G245S,G152S,G113S | No | No |
| DO51517 | Male | PDAC | 52.0 | IIB | G3 | Relapse | Distant recurrence/metastasis | Deceased | 1389.0 | No | No | No | No |
| DO51518 | Female | PDAC | 66.0 | IIB | G3 | Relapse | Distant recurrence/metastasis | Deceased | 129.0 | G12D | R248W,R116W,R155W | No | No |
| DO51519 | Female | PDAC | 70.0 | IIB | G3 | Progression | Distant recurrence/metastasis | Deceased | 579.0 | G12D | No | No | No |
| DO51520 | NA | PDAC | 73.0 | IIB | G2 | Complete remission | NA | Deceased | 139.0 | G12D | No | No | No |
| DO51521 | Female | Carcinoma, NOS | 78.0 | IIB | G3 | NA | NA | NA | 272.0 | No | No | No | No |
| DO51522 | Female | PDAC | 50.0 | IIA | G2 | Relapse | Local recurrence and distant metastasis | Deceased | 201.0 | G12D | No | No | No |
| DO51523 | Male | Adenocarcinoma, NOS | 48.0 | IB | G3 | Relapse | Distant recurrence/metastasis | Deceased | 318.0 | G12D | V274A,V142A | No | No |
| DO51524 | Male | PDAC | 79.0 | IIA | G1 | Complete remission | NA | Alive | 1251.0 | G12D | No | No | R361H,R265H |
| DO51525 | Male | PDAC | 38.0 | IIB | G2 | Relapse | Local recurrence and distant metastasis | Deceased | 690.0 | G12D,G12R | No | No | No |
| DO51526 | Female | PDAC | 82.0 | IIB | G2 | Relapse | Distant recurrence/metastasis | Deceased | 1380.0 | G12V | No | No | No |
| DO51527 | Female | PDAC | 86.0 | IIB | G2 | Complete remission | NA | Deceased | 349.0 | G12D | No | No | No |
| DO51528 | Male | Adenocarcinoma, NOS | 62.0 | IIB | G4 | Complete remission | NA | Alive | 2325.0 | G12V | C238R,C145R,C106R | No | No |
| DO51529 | Male | Carcinoma, NOS | 87.0 | IIB | G2 | Relapse | NA | Deceased | 276.0 | G12R | R282W,R150W | No | No |
| DO51530 | Female | PDAC | 43.0 | IIA | G2 | Relapse | Distant recurrence/metastasis | Deceased | 625.0 | G12R | No | No | No |
| DO51531 | Male | PDAC | 48.0 | IIB | G3 | Relapse | NA | Deceased | 1273.0 | G12D | V272L,V140L | No | No |
| DO51532 | Female | PDAC | 59.0 | IIB | G1 | Relapse | Distant recurrence/metastasis | Alive | 1761.0 | G12V | R175H,R82H,R43H | No | No |
| DO51533 | Male | Adenocarcinoma, NOS | 67.0 | IB | G3 | Complete remission | NA | Alive | 4173.0 | G12V | No | No | No |
| DO51534 | Female | PDAC | 79.0 | IIB | G2 | Relapse | Distant recurrence/metastasis | Deceased | 278.0 | G12V | No | P94L,P135L | R361C,R265C |
| DO51535 | Male | Mucinous adenocarcinoma | 84.0 | IIB | G3 | Progression | Distant recurrence/metastasis | Deceased | 764.0 | G12V | A161T,A29T,A68T | No | No |
| DO51536 | Female | PDAC | 82.0 | IIB | G3 | Relapse | NA | Deceased | 446.0 | G12R | No | No | No |
| DO51537 | Male | PDAC | 73.0 | IIB | G2 | Relapse | Local recurrence and distant metastasis | Deceased | 503.0 | G12D | P151A,P58A,P19A | No | No |
| DO51538 | Female | PDAC | 76.0 | IIB | G2 | Relapse | Local recurrence and distant metastasis | Deceased | 361.0 | G12D | H86Y,H179Y,H47Y | No | No |
| DO51540 | Female | Adenocarcinoma, NOS | 69.0 | IIA | G4 | Relapse | Distant recurrence/metastasis | Deceased | 141.0 | G12D | R273C,R141C | No | No |
| DO51541 | Male | Adenocarcinoma, NOS | 81.0 | IIB | G4 | Relapse | Distant recurrence/metastasis | Deceased | 325.0 | G12V | M114V,M246V,M153V,G245R,G113R,G152R | No | No |
| DO51542 | Female | PDAC | 54.0 | IIB | G2 | Progression | Local recurrence and distant metastasis | Deceased | 369.0 | G12D | K132R,K39R | No | No |
| DO51543 | Female | PDAC | 76.0 | IIB | G2 | Complete remission | NA | Deceased | 1431.0 | G12R | M105I,M237I,M144I | No | R361H,R265H |
| DO51544 | Male | Adenocarcinoma, NOS | 69.0 | IIB | G3 | NA | NA | Deceased | 234.0 | G12D | Y220C,Y127C,Y88C | No | No |
| DO51545 | Female | PDAC | 75.0 | IIB | G3 | Complete remission | NA | Deceased | 190.0 | G12D | No | No | No |
| DO51546 | Female | PDAC | 73.0 | IIB | G2 | Complete remission | NA | Deceased | 220.0 | G12V | Q192H,Q99H,Q60H,H193Y,H61Y,H100Y | No | No |
| DO51548 | Female | PDAC | 55.0 | IIA | G2 | Complete remission | NA | Alive | 2177.0 | G12V | No | No | R361C,R265C |
| DO51549 | Female | PDAC | 76.0 | IIB | G2 | Complete remission | NA | Alive | 229.0 | G12V | No | No | No |
Showing 1 to 317 of 317 entries
Processing...
Overview
Patient Statistics
Tumour Statistics
Cohort Summary
Variant Identification
Drug Prediction
Cancer Genome Interpreter (CGI) help
25%
| Gene | Protein Alteration | Alteration Match | Drug | Effect | Tumour | Evidence |
|---|
Gene | Protein Alteration | Alteration Match | Drug | Effect | Tumour | Evidence |
|---|---|---|---|---|---|---|
| ABL1 | intron_variant | Different Alteration | Ponatinib (BCR-ABL inhibitor & Pan-TK inhibitor) | Responsive | CML, ALL | FDA guidelines |
| ABL1 | intron_variant | Different Alteration | Dasatinib (BCR-ABL inhibitor) | Responsive | ALL, CML | FDA guidelines |
| ABL1 | intron_variant | Different Alteration | Nilotinib (BCR-ABL inhibitor) | Responsive | CML | FDA guidelines |
| ABL1 | intron_variant | Different Alteration | Imatinib (BCR-ABL inhibitor & KIT inhibitor) | Responsive | ALL, CML | FDA guidelines |
| ABL1 | intron_variant | Different Alteration | Dasatinib;Venetoclax (BCR-ABL inhibitor;BCL2 inhibitor) | Responsive | ALL | Pre-clinical |
| ABL1 | intron_variant | Different Alteration | Bosutinib (BCR-ABL inhibitor) | Responsive | CML | FDA guidelines |
| ABL1 | intron_variant), ABL1 MUT* (intron_variant | Different Alteration | Ponatinib (BCR-ABL inhibitor & Pan-TK inhibitor) | Responsive | CML, ALL | FDA guidelines |
| ABL1 | intron_variant), ABL1 MUT* (intron_variant | Different Alteration | Dasatinib (BCR-ABL inhibitor) | Responsive | ALL, CML | FDA guidelines |
| ABL1 | intron_variant), ABL1 MUT* (intron_variant | Different Alteration | Nilotinib (BCR-ABL inhibitor) | Responsive | CML | FDA guidelines |
| ABL1 | intron_variant), ABL1 MUT* (intron_variant | Different Alteration | Imatinib (BCR-ABL inhibitor & KIT inhibitor) | Responsive | ALL, CML | FDA guidelines |
| ABL1 | intron_variant), ABL1 MUT* (intron_variant | Different Alteration | Dasatinib;Venetoclax (BCR-ABL inhibitor;BCL2 inhibitor) | Responsive | ALL | Pre-clinical |
| ABL1 | intron_variant), ABL1 MUT* (intron_variant | Different Alteration | Bosutinib (BCR-ABL inhibitor) | Responsive | CML | FDA guidelines |
| ABL1 | 3-UTRSNV), ABL1 MUT* (intron_variant), ABL1 MUT* (IntronicBlockSubstitution), ABL1 MUT* (IntronicBlockSubstitution | Different Alteration | Ponatinib (BCR-ABL inhibitor & Pan-TK inhibitor) | Responsive | CML, ALL | FDA guidelines |
| ABL1 | 3-UTRSNV), ABL1 MUT* (intron_variant), ABL1 MUT* (IntronicBlockSubstitution), ABL1 MUT* (IntronicBlockSubstitution | Different Alteration | Dasatinib (BCR-ABL inhibitor) | Responsive | ALL, CML | FDA guidelines |
| ABL1 | 3-UTRSNV), ABL1 MUT* (intron_variant), ABL1 MUT* (IntronicBlockSubstitution), ABL1 MUT* (IntronicBlockSubstitution | Different Alteration | Nilotinib (BCR-ABL inhibitor) | Responsive | CML | FDA guidelines |
| ABL1 | 3-UTRSNV), ABL1 MUT* (intron_variant), ABL1 MUT* (IntronicBlockSubstitution), ABL1 MUT* (IntronicBlockSubstitution | Different Alteration | Imatinib (BCR-ABL inhibitor & KIT inhibitor) | Responsive | ALL, CML | FDA guidelines |
| ABL1 | 3-UTRSNV), ABL1 MUT* (intron_variant), ABL1 MUT* (IntronicBlockSubstitution), ABL1 MUT* (IntronicBlockSubstitution | Different Alteration | Dasatinib;Venetoclax (BCR-ABL inhibitor;BCL2 inhibitor) | Responsive | ALL | Pre-clinical |
| ABL1 | 3-UTRSNV), ABL1 MUT* (intron_variant), ABL1 MUT* (IntronicBlockSubstitution), ABL1 MUT* (IntronicBlockSubstitution | Different Alteration | Bosutinib (BCR-ABL inhibitor) | Responsive | CML | FDA guidelines |
| ABL1 | 3-UTRBlockSubstitution), ABL1 MUT* (3-UTRSNV | Different Alteration | Ponatinib (BCR-ABL inhibitor & Pan-TK inhibitor) | Responsive | CML, ALL | FDA guidelines |
| ABL1 | 3-UTRBlockSubstitution), ABL1 MUT* (3-UTRSNV | Different Alteration | Dasatinib (BCR-ABL inhibitor) | Responsive | ALL, CML | FDA guidelines |
| ABL1 | 3-UTRBlockSubstitution), ABL1 MUT* (3-UTRSNV | Different Alteration | Nilotinib (BCR-ABL inhibitor) | Responsive | CML | FDA guidelines |
| ABL1 | 3-UTRBlockSubstitution), ABL1 MUT* (3-UTRSNV | Different Alteration | Imatinib (BCR-ABL inhibitor & KIT inhibitor) | Responsive | ALL, CML | FDA guidelines |
| ABL1 | 3-UTRBlockSubstitution), ABL1 MUT* (3-UTRSNV | Different Alteration | Dasatinib;Venetoclax (BCR-ABL inhibitor;BCL2 inhibitor) | Responsive | ALL | Pre-clinical |
| ABL1 | 3-UTRBlockSubstitution), ABL1 MUT* (3-UTRSNV | Different Alteration | Bosutinib (BCR-ABL inhibitor) | Responsive | CML | FDA guidelines |
| ABL1 | intron_variant), ABL1 MUT* (intron_variant), ABL1 MUT* (intron_variant | Different Alteration | Ponatinib (BCR-ABL inhibitor & Pan-TK inhibitor) | Responsive | CML, ALL | FDA guidelines |
| ABL1 | intron_variant), ABL1 MUT* (intron_variant), ABL1 MUT* (intron_variant | Different Alteration | Dasatinib (BCR-ABL inhibitor) | Responsive | ALL, CML | FDA guidelines |
| ABL1 | intron_variant), ABL1 MUT* (intron_variant), ABL1 MUT* (intron_variant | Different Alteration | Nilotinib (BCR-ABL inhibitor) | Responsive | CML | FDA guidelines |
| ABL1 | intron_variant), ABL1 MUT* (intron_variant), ABL1 MUT* (intron_variant | Different Alteration | Imatinib (BCR-ABL inhibitor & KIT inhibitor) | Responsive | ALL, CML | FDA guidelines |
| ABL1 | intron_variant), ABL1 MUT* (intron_variant), ABL1 MUT* (intron_variant | Different Alteration | Dasatinib;Venetoclax (BCR-ABL inhibitor;BCL2 inhibitor) | Responsive | ALL | Pre-clinical |
| ABL1 | intron_variant), ABL1 MUT* (intron_variant), ABL1 MUT* (intron_variant | Different Alteration | Bosutinib (BCR-ABL inhibitor) | Responsive | CML | FDA guidelines |
| AKT3 | intron_variant), AKT3 MUT* (intron_variant | Different Alteration | ATP competitive AKT inhibitor | Responsive | BRCA | Pre-clinical |
| AKT3 | intron_variant), AKT3 MUT* (intron_variant), AKT3 MUT* (intron_variant), AKT3 MUT* (intron_variant | Different Alteration | ATP competitive AKT inhibitor | Responsive | BRCA | Pre-clinical |
| AKT3 | intron_variant | Different Alteration | ATP competitive AKT inhibitor | Responsive | BRCA | Pre-clinical |
| AKT3 | intron_variant), AKT3 MUT* (intron_variant), AKT3 MUT* (intron_variant | Different Alteration | ATP competitive AKT inhibitor | Responsive | BRCA | Pre-clinical |
| AKT3 | intron_variant), AKT3 MUT* (intron_variant), AKT3 MUT* (intron_variant), AKT3 MUT* (intron_variant), AKT3 MUT* (intron_variant), AKT3 MUT* (intron_variant), AKT3 MUT* (intron_variant), AKT3 MUT* (intron_variant), AKT3 MUT* (intron_variant), AKT3 MUT* | Different Alteration | ATP competitive AKT inhibitor | Responsive | BRCA | Pre-clinical |
| AKT3 | intron_variant), AKT3 MUT* (intron_variant), AKT3 MUT* (intron_variant), AKT3 MUT* (intron_variant), AKT3 MUT* (intron_variant), AKT3 MUT* (intron_variant), AKT3 MUT* (intron_variant), AKT3 MUT* (intron_variant | Different Alteration | ATP competitive AKT inhibitor | Responsive | BRCA | Pre-clinical |
| AKT3 | intron_variant), AKT3 MUT* (intron_variant), AKT3 MUT* (intron_variant), AKT3 MUT* (intron_variant), AKT3 MUT* (intron_variant), AKT3 MUT* (intron_variant | Different Alteration | ATP competitive AKT inhibitor | Responsive | BRCA | Pre-clinical |
| AKT3 | N21H | Different Alteration | ATP competitive AKT inhibitor | Responsive | BRCA | Pre-clinical |
| AKT3 | intron_variant), AKT3 MUT* (intron_variant), AKT3 MUT* (intron_variant), AKT3 MUT* (intron_variant), AKT3 MUT* (intron_variant), AKT3 MUT* (intron_variant), AKT3 MUT* (intron_variant | Different Alteration | ATP competitive AKT inhibitor | Responsive | BRCA | Pre-clinical |
| AKT3 | intron_variant), AKT3 MUT* (intron_variant), AKT3 MUT* (intron_variant), AKT3 MUT* (intron_variant), AKT3 MUT* (intron_variant | Different Alteration | ATP competitive AKT inhibitor | Responsive | BRCA | Pre-clinical |
| AKT3 | E232K | Different Alteration | ATP competitive AKT inhibitor | Responsive | BRCA | Pre-clinical |
| AKT3 | intron_variant), AKT3 MUT* (intron_variant), AKT3 MUT* (intron_variant), AKT3 MUT* (intron_variant), AKT3 MUT* (intron_variant), AKT3 MUT* (intron_variant), AKT3 MUT* (intron_variant), AKT3 MUT* (intron_variant), AKT3 MUT* (intron_variant | Different Alteration | ATP competitive AKT inhibitor | Responsive | BRCA | Pre-clinical |
| AKT3 | intron_variant), AKT3 MUT* (intron_variant), AKT3 MUT* (intron_variant), AKT3 MUT* (intron_variant), AKT3 MUT* (intron_variant), AKT3 MUT* (intron_variant), AKT3 MUT* (intron_variant), AKT3 MUT* (intron_variant), AKT3 MUT* (3-UTRSNV | Different Alteration | ATP competitive AKT inhibitor | Responsive | BRCA | Pre-clinical |
| ALK | intron_variant | Different Alteration | ALK inhibitor;SRC inhibitor | Responsive | LUAD | Pre-clinical |
| ALK | intron_variant | Different Alteration | Crizotinib (ALK inhibitor) | Responsive | GB, LY | Early trials |
| ALK | intron_variant | Different Alteration | Lorlatinib (ALK&ROS1 inhibitor) | Responsive | NSCLC | Early trials |
| ALK | intron_variant | Different Alteration | Crizotinib (ALK inhibitor) | Responsive | THCA, IM | Case report |
| ALK | intron_variant | Different Alteration | HSP90 inhibitor | Responsive | LUAD | Early trials |
| ALK | intron_variant | Different Alteration | Ceritinib (ALK inhibitor) | Responsive | LUAD, NSCLC | FDA guidelines |
| ALK | intron_variant | Different Alteration | Ceritinib (ALK inhibitor) | Responsive | IM, COREAD | Case report |
| ALK | intron_variant | Different Alteration | Crizotinib (ALK inhibitor) | Responsive | LUAD, NSCLC | FDA guidelines |
| ALK | intron_variant | Different Alteration | Brigatinib (Pan-TK inhibitor) | Responsive | NSCLC | FDA guidelines |
| ALK | intron_variant | Different Alteration | Entrictinib (Pan-TK inhibitor) | Responsive | COREAD | Case report |
| ALK | intron_variant | Different Alteration | Alectinib (ALK inhibitor) | Responsive | LUAD | FDA guidelines |
| ALK | intron_variant | Different Alteration | HSP90 inhibitor (HSP90 inhibitor) | Responsive | LUAD | Early trials |
| ALK | intron_variant | Different Alteration | ALK inhibitor;IGF1R inhibitor | Responsive | LUAD | Pre-clinical |
| ALK | intron_variant | Different Alteration | ALK inhibitor;MEK inhibitor | Responsive | LUAD | Pre-clinical |
| ALK | intron_variant | Different Alteration | novel ALK inhibitor | Responsive | LUAD | Early trials |
| ALK | intron_variant | Different Alteration | Ceritinib (ALK inhibitor) | Responsive | HEMATO | Early trials |
| ALK | intron_variant | Different Alteration | ALK inhibitor | Responsive | COREAD | Case report |
| ALK | intron_variant | Different Alteration | Alectinib (ALK inhibitor) | Responsive | NSCLC | FDA guidelines |
| ALK | intron_variant | Different Alteration | MEK inhibitor (ALK inhibitor;MEK inhibitor) | Responsive | LUAD | Pre-clinical |
| ALK | intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant | Different Alteration | ALK inhibitor;SRC inhibitor | Responsive | LUAD | Pre-clinical |
| ALK | intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant | Different Alteration | Crizotinib (ALK inhibitor) | Responsive | GB, LY | Early trials |
| ALK | intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant | Different Alteration | Lorlatinib (ALK&ROS1 inhibitor) | Responsive | NSCLC | Early trials |
| ALK | intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant | Different Alteration | Crizotinib (ALK inhibitor) | Responsive | THCA, IM | Case report |
| ALK | intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant | Different Alteration | HSP90 inhibitor | Responsive | LUAD | Early trials |
| ALK | intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant | Different Alteration | Ceritinib (ALK inhibitor) | Responsive | LUAD, NSCLC | FDA guidelines |
| ALK | intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant | Different Alteration | Ceritinib (ALK inhibitor) | Responsive | IM, COREAD | Case report |
| ALK | intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant | Different Alteration | Crizotinib (ALK inhibitor) | Responsive | LUAD, NSCLC | FDA guidelines |
| ALK | intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant | Different Alteration | Brigatinib (Pan-TK inhibitor) | Responsive | NSCLC | FDA guidelines |
| ALK | intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant | Different Alteration | Entrictinib (Pan-TK inhibitor) | Responsive | COREAD | Case report |
| ALK | intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant | Different Alteration | Alectinib (ALK inhibitor) | Responsive | LUAD | FDA guidelines |
| ALK | intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant | Different Alteration | HSP90 inhibitor (HSP90 inhibitor) | Responsive | LUAD | Early trials |
| ALK | intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant | Different Alteration | ALK inhibitor;IGF1R inhibitor | Responsive | LUAD | Pre-clinical |
| ALK | intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant | Different Alteration | ALK inhibitor;MEK inhibitor | Responsive | LUAD | Pre-clinical |
| ALK | intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant | Different Alteration | novel ALK inhibitor | Responsive | LUAD | Early trials |
| ALK | intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant | Different Alteration | Ceritinib (ALK inhibitor) | Responsive | HEMATO | Early trials |
| ALK | intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant | Different Alteration | ALK inhibitor | Responsive | COREAD | Case report |
| ALK | intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant | Different Alteration | Alectinib (ALK inhibitor) | Responsive | NSCLC | FDA guidelines |
| ALK | intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant | Different Alteration | MEK inhibitor (ALK inhibitor;MEK inhibitor) | Responsive | LUAD | Pre-clinical |
| ALK | intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant | Different Alteration | ALK inhibitor;SRC inhibitor | Responsive | LUAD | Pre-clinical |
| ALK | intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant | Different Alteration | Crizotinib (ALK inhibitor) | Responsive | GB, LY | Early trials |
| ALK | intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant | Different Alteration | Lorlatinib (ALK&ROS1 inhibitor) | Responsive | NSCLC | Early trials |
| ALK | intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant | Different Alteration | Crizotinib (ALK inhibitor) | Responsive | THCA, IM | Case report |
| ALK | intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant | Different Alteration | HSP90 inhibitor | Responsive | LUAD | Early trials |
| ALK | intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant | Different Alteration | Ceritinib (ALK inhibitor) | Responsive | LUAD, NSCLC | FDA guidelines |
| ALK | intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant | Different Alteration | Ceritinib (ALK inhibitor) | Responsive | IM, COREAD | Case report |
| ALK | intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant | Different Alteration | Crizotinib (ALK inhibitor) | Responsive | LUAD, NSCLC | FDA guidelines |
| ALK | intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant | Different Alteration | Brigatinib (Pan-TK inhibitor) | Responsive | NSCLC | FDA guidelines |
| ALK | intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant | Different Alteration | Entrictinib (Pan-TK inhibitor) | Responsive | COREAD | Case report |
| ALK | intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant | Different Alteration | Alectinib (ALK inhibitor) | Responsive | LUAD | FDA guidelines |
| ALK | intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant | Different Alteration | HSP90 inhibitor (HSP90 inhibitor) | Responsive | LUAD | Early trials |
| ALK | intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant | Different Alteration | ALK inhibitor;IGF1R inhibitor | Responsive | LUAD | Pre-clinical |
| ALK | intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant | Different Alteration | ALK inhibitor;MEK inhibitor | Responsive | LUAD | Pre-clinical |
| ALK | intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant | Different Alteration | novel ALK inhibitor | Responsive | LUAD | Early trials |
| ALK | intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant | Different Alteration | Ceritinib (ALK inhibitor) | Responsive | HEMATO | Early trials |
| ALK | intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant | Different Alteration | ALK inhibitor | Responsive | COREAD | Case report |
| ALK | intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant | Different Alteration | Alectinib (ALK inhibitor) | Responsive | NSCLC | FDA guidelines |
| ALK | intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant | Different Alteration | MEK inhibitor (ALK inhibitor;MEK inhibitor) | Responsive | LUAD | Pre-clinical |
| ALK | intron_variant), ALK MUT* (intron_variant | Different Alteration | ALK inhibitor;SRC inhibitor | Responsive | LUAD | Pre-clinical |
| ALK | intron_variant), ALK MUT* (intron_variant | Different Alteration | Crizotinib (ALK inhibitor) | Responsive | GB, LY | Early trials |
| ALK | intron_variant), ALK MUT* (intron_variant | Different Alteration | Lorlatinib (ALK&ROS1 inhibitor) | Responsive | NSCLC | Early trials |
| ALK | intron_variant), ALK MUT* (intron_variant | Different Alteration | Crizotinib (ALK inhibitor) | Responsive | THCA, IM | Case report |
| ALK | intron_variant), ALK MUT* (intron_variant | Different Alteration | HSP90 inhibitor | Responsive | LUAD | Early trials |
| ALK | intron_variant), ALK MUT* (intron_variant | Different Alteration | Ceritinib (ALK inhibitor) | Responsive | LUAD, NSCLC | FDA guidelines |
| ALK | intron_variant), ALK MUT* (intron_variant | Different Alteration | Ceritinib (ALK inhibitor) | Responsive | IM, COREAD | Case report |
| ALK | intron_variant), ALK MUT* (intron_variant | Different Alteration | Crizotinib (ALK inhibitor) | Responsive | LUAD, NSCLC | FDA guidelines |
| ALK | intron_variant), ALK MUT* (intron_variant | Different Alteration | Brigatinib (Pan-TK inhibitor) | Responsive | NSCLC | FDA guidelines |
| ALK | intron_variant), ALK MUT* (intron_variant | Different Alteration | Entrictinib (Pan-TK inhibitor) | Responsive | COREAD | Case report |
| ALK | intron_variant), ALK MUT* (intron_variant | Different Alteration | Alectinib (ALK inhibitor) | Responsive | LUAD | FDA guidelines |
| ALK | intron_variant), ALK MUT* (intron_variant | Different Alteration | HSP90 inhibitor (HSP90 inhibitor) | Responsive | LUAD | Early trials |
| ALK | intron_variant), ALK MUT* (intron_variant | Different Alteration | ALK inhibitor;IGF1R inhibitor | Responsive | LUAD | Pre-clinical |
| ALK | intron_variant), ALK MUT* (intron_variant | Different Alteration | ALK inhibitor;MEK inhibitor | Responsive | LUAD | Pre-clinical |
| ALK | intron_variant), ALK MUT* (intron_variant | Different Alteration | novel ALK inhibitor | Responsive | LUAD | Early trials |
| ALK | intron_variant), ALK MUT* (intron_variant | Different Alteration | Ceritinib (ALK inhibitor) | Responsive | HEMATO | Early trials |
| ALK | intron_variant), ALK MUT* (intron_variant | Different Alteration | ALK inhibitor | Responsive | COREAD | Case report |
| ALK | intron_variant), ALK MUT* (intron_variant | Different Alteration | Alectinib (ALK inhibitor) | Responsive | NSCLC | FDA guidelines |
| ALK | intron_variant), ALK MUT* (intron_variant | Different Alteration | MEK inhibitor (ALK inhibitor;MEK inhibitor) | Responsive | LUAD | Pre-clinical |
| ALK | intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant | Different Alteration | ALK inhibitor;SRC inhibitor | Responsive | LUAD | Pre-clinical |
| ALK | intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant | Different Alteration | Crizotinib (ALK inhibitor) | Responsive | GB, LY | Early trials |
| ALK | intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant | Different Alteration | Lorlatinib (ALK&ROS1 inhibitor) | Responsive | NSCLC | Early trials |
| ALK | intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant | Different Alteration | Crizotinib (ALK inhibitor) | Responsive | THCA, IM | Case report |
| ALK | intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant | Different Alteration | HSP90 inhibitor | Responsive | LUAD | Early trials |
| ALK | intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant | Different Alteration | Ceritinib (ALK inhibitor) | Responsive | LUAD, NSCLC | FDA guidelines |
| ALK | intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant | Different Alteration | Ceritinib (ALK inhibitor) | Responsive | IM, COREAD | Case report |
| ALK | intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant | Different Alteration | Crizotinib (ALK inhibitor) | Responsive | LUAD, NSCLC | FDA guidelines |
| ALK | intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant | Different Alteration | Brigatinib (Pan-TK inhibitor) | Responsive | NSCLC | FDA guidelines |
| ALK | intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant | Different Alteration | Entrictinib (Pan-TK inhibitor) | Responsive | COREAD | Case report |
| ALK | intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant | Different Alteration | Alectinib (ALK inhibitor) | Responsive | LUAD | FDA guidelines |
| ALK | intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant | Different Alteration | HSP90 inhibitor (HSP90 inhibitor) | Responsive | LUAD | Early trials |
| ALK | intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant | Different Alteration | ALK inhibitor;IGF1R inhibitor | Responsive | LUAD | Pre-clinical |
| ALK | intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant | Different Alteration | ALK inhibitor;MEK inhibitor | Responsive | LUAD | Pre-clinical |
| ALK | intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant | Different Alteration | novel ALK inhibitor | Responsive | LUAD | Early trials |
| ALK | intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant | Different Alteration | Ceritinib (ALK inhibitor) | Responsive | HEMATO | Early trials |
| ALK | intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant | Different Alteration | ALK inhibitor | Responsive | COREAD | Case report |
| ALK | intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant | Different Alteration | Alectinib (ALK inhibitor) | Responsive | NSCLC | FDA guidelines |
| ALK | intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant | Different Alteration | MEK inhibitor (ALK inhibitor;MEK inhibitor) | Responsive | LUAD | Pre-clinical |
| ALK | intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant | Different Alteration | ALK inhibitor;SRC inhibitor | Responsive | LUAD | Pre-clinical |
| ALK | intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant | Different Alteration | Crizotinib (ALK inhibitor) | Responsive | GB, LY | Early trials |
| ALK | intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant | Different Alteration | Lorlatinib (ALK&ROS1 inhibitor) | Responsive | NSCLC | Early trials |
| ALK | intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant | Different Alteration | Crizotinib (ALK inhibitor) | Responsive | THCA, IM | Case report |
| ALK | intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant | Different Alteration | HSP90 inhibitor | Responsive | LUAD | Early trials |
| ALK | intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant | Different Alteration | Ceritinib (ALK inhibitor) | Responsive | LUAD, NSCLC | FDA guidelines |
| ALK | intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant | Different Alteration | Ceritinib (ALK inhibitor) | Responsive | IM, COREAD | Case report |
| ALK | intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant | Different Alteration | Crizotinib (ALK inhibitor) | Responsive | LUAD, NSCLC | FDA guidelines |
| ALK | intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant | Different Alteration | Brigatinib (Pan-TK inhibitor) | Responsive | NSCLC | FDA guidelines |
| ALK | intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant | Different Alteration | Entrictinib (Pan-TK inhibitor) | Responsive | COREAD | Case report |
| ALK | intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant | Different Alteration | Alectinib (ALK inhibitor) | Responsive | LUAD | FDA guidelines |
| ALK | intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant | Different Alteration | HSP90 inhibitor (HSP90 inhibitor) | Responsive | LUAD | Early trials |
| ALK | intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant | Different Alteration | ALK inhibitor;IGF1R inhibitor | Responsive | LUAD | Pre-clinical |
| ALK | intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant | Different Alteration | ALK inhibitor;MEK inhibitor | Responsive | LUAD | Pre-clinical |
| ALK | intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant | Different Alteration | novel ALK inhibitor | Responsive | LUAD | Early trials |
| ALK | intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant | Different Alteration | Ceritinib (ALK inhibitor) | Responsive | HEMATO | Early trials |
| ALK | intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant | Different Alteration | ALK inhibitor | Responsive | COREAD | Case report |
| ALK | intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant | Different Alteration | Alectinib (ALK inhibitor) | Responsive | NSCLC | FDA guidelines |
| ALK | intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant | Different Alteration | MEK inhibitor (ALK inhibitor;MEK inhibitor) | Responsive | LUAD | Pre-clinical |
| ALK | intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant | Different Alteration | ALK inhibitor;SRC inhibitor | Responsive | LUAD | Pre-clinical |
| ALK | intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant | Different Alteration | Crizotinib (ALK inhibitor) | Responsive | GB, LY | Early trials |
| ALK | intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant | Different Alteration | Lorlatinib (ALK&ROS1 inhibitor) | Responsive | NSCLC | Early trials |
| ALK | intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant | Different Alteration | Crizotinib (ALK inhibitor) | Responsive | THCA, IM | Case report |
| ALK | intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant | Different Alteration | HSP90 inhibitor | Responsive | LUAD | Early trials |
| ALK | intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant | Different Alteration | Ceritinib (ALK inhibitor) | Responsive | LUAD, NSCLC | FDA guidelines |
| ALK | intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant | Different Alteration | Ceritinib (ALK inhibitor) | Responsive | IM, COREAD | Case report |
| ALK | intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant | Different Alteration | Crizotinib (ALK inhibitor) | Responsive | LUAD, NSCLC | FDA guidelines |
| ALK | intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant | Different Alteration | Brigatinib (Pan-TK inhibitor) | Responsive | NSCLC | FDA guidelines |
| ALK | intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant | Different Alteration | Entrictinib (Pan-TK inhibitor) | Responsive | COREAD | Case report |
| ALK | intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant | Different Alteration | Alectinib (ALK inhibitor) | Responsive | LUAD | FDA guidelines |
| ALK | intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant | Different Alteration | HSP90 inhibitor (HSP90 inhibitor) | Responsive | LUAD | Early trials |
| ALK | intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant | Different Alteration | ALK inhibitor;IGF1R inhibitor | Responsive | LUAD | Pre-clinical |
| ALK | intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant | Different Alteration | ALK inhibitor;MEK inhibitor | Responsive | LUAD | Pre-clinical |
| ALK | intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant | Different Alteration | novel ALK inhibitor | Responsive | LUAD | Early trials |
| ALK | intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant | Different Alteration | Ceritinib (ALK inhibitor) | Responsive | HEMATO | Early trials |
| ALK | intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant | Different Alteration | ALK inhibitor | Responsive | COREAD | Case report |
| ALK | intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant | Different Alteration | Alectinib (ALK inhibitor) | Responsive | NSCLC | FDA guidelines |
| ALK | intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant | Different Alteration | MEK inhibitor (ALK inhibitor;MEK inhibitor) | Responsive | LUAD | Pre-clinical |
| ALK | intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_ | Different Alteration | ALK inhibitor;SRC inhibitor | Responsive | LUAD | Pre-clinical |
| ALK | intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_ | Different Alteration | Crizotinib (ALK inhibitor) | Responsive | GB, LY | Early trials |
| ALK | intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_ | Different Alteration | Lorlatinib (ALK&ROS1 inhibitor) | Responsive | NSCLC | Early trials |
| ALK | intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_ | Different Alteration | Crizotinib (ALK inhibitor) | Responsive | THCA, IM | Case report |
| ALK | intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_ | Different Alteration | HSP90 inhibitor | Responsive | LUAD | Early trials |
| ALK | intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_ | Different Alteration | Ceritinib (ALK inhibitor) | Responsive | LUAD, NSCLC | FDA guidelines |
| ALK | intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_ | Different Alteration | Ceritinib (ALK inhibitor) | Responsive | IM, COREAD | Case report |
| ALK | intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_ | Different Alteration | Crizotinib (ALK inhibitor) | Responsive | LUAD, NSCLC | FDA guidelines |
| ALK | intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_ | Different Alteration | Brigatinib (Pan-TK inhibitor) | Responsive | NSCLC | FDA guidelines |
| ALK | intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_ | Different Alteration | Entrictinib (Pan-TK inhibitor) | Responsive | COREAD | Case report |
| ALK | intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_ | Different Alteration | Alectinib (ALK inhibitor) | Responsive | LUAD | FDA guidelines |
| ALK | intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_ | Different Alteration | HSP90 inhibitor (HSP90 inhibitor) | Responsive | LUAD | Early trials |
| ALK | intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_ | Different Alteration | ALK inhibitor;IGF1R inhibitor | Responsive | LUAD | Pre-clinical |
| ALK | intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_ | Different Alteration | ALK inhibitor;MEK inhibitor | Responsive | LUAD | Pre-clinical |
| ALK | intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_ | Different Alteration | novel ALK inhibitor | Responsive | LUAD | Early trials |
| ALK | intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_ | Different Alteration | Ceritinib (ALK inhibitor) | Responsive | HEMATO | Early trials |
| ALK | intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_ | Different Alteration | ALK inhibitor | Responsive | COREAD | Case report |
| ALK | intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_ | Different Alteration | Alectinib (ALK inhibitor) | Responsive | NSCLC | FDA guidelines |
| ALK | intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_ | Different Alteration | MEK inhibitor (ALK inhibitor;MEK inhibitor) | Responsive | LUAD | Pre-clinical |
| ALK | intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant | Different Alteration | ALK inhibitor;SRC inhibitor | Responsive | LUAD | Pre-clinical |
| ALK | intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant | Different Alteration | Crizotinib (ALK inhibitor) | Responsive | GB, LY | Early trials |
| ALK | intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant | Different Alteration | Lorlatinib (ALK&ROS1 inhibitor) | Responsive | NSCLC | Early trials |
| ALK | intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant | Different Alteration | Crizotinib (ALK inhibitor) | Responsive | THCA, IM | Case report |
| ALK | intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant | Different Alteration | HSP90 inhibitor | Responsive | LUAD | Early trials |
| ALK | intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant | Different Alteration | Ceritinib (ALK inhibitor) | Responsive | LUAD, NSCLC | FDA guidelines |
| ALK | intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant | Different Alteration | Ceritinib (ALK inhibitor) | Responsive | IM, COREAD | Case report |
| ALK | intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant | Different Alteration | Crizotinib (ALK inhibitor) | Responsive | LUAD, NSCLC | FDA guidelines |
| ALK | intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant | Different Alteration | Brigatinib (Pan-TK inhibitor) | Responsive | NSCLC | FDA guidelines |
| ALK | intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant | Different Alteration | Entrictinib (Pan-TK inhibitor) | Responsive | COREAD | Case report |
| ALK | intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant | Different Alteration | Alectinib (ALK inhibitor) | Responsive | LUAD | FDA guidelines |
| ALK | intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant | Different Alteration | HSP90 inhibitor (HSP90 inhibitor) | Responsive | LUAD | Early trials |
| ALK | intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant | Different Alteration | ALK inhibitor;IGF1R inhibitor | Responsive | LUAD | Pre-clinical |
| ALK | intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant | Different Alteration | ALK inhibitor;MEK inhibitor | Responsive | LUAD | Pre-clinical |
| ALK | intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant | Different Alteration | novel ALK inhibitor | Responsive | LUAD | Early trials |
| ALK | intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant | Different Alteration | Ceritinib (ALK inhibitor) | Responsive | HEMATO | Early trials |
| ALK | intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant | Different Alteration | ALK inhibitor | Responsive | COREAD | Case report |
| ALK | intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant | Different Alteration | Alectinib (ALK inhibitor) | Responsive | NSCLC | FDA guidelines |
| ALK | intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant | Different Alteration | MEK inhibitor (ALK inhibitor;MEK inhibitor) | Responsive | LUAD | Pre-clinical |
| ALK | 5-UTRSNV | Different Alteration | ALK inhibitor;SRC inhibitor | Responsive | LUAD | Pre-clinical |
| ALK | 5-UTRSNV | Different Alteration | Crizotinib (ALK inhibitor) | Responsive | GB, LY | Early trials |
| ALK | 5-UTRSNV | Different Alteration | Lorlatinib (ALK&ROS1 inhibitor) | Responsive | NSCLC | Early trials |
| ALK | 5-UTRSNV | Different Alteration | Crizotinib (ALK inhibitor) | Responsive | THCA, IM | Case report |
| ALK | 5-UTRSNV | Different Alteration | HSP90 inhibitor | Responsive | LUAD | Early trials |
| ALK | 5-UTRSNV | Different Alteration | Ceritinib (ALK inhibitor) | Responsive | LUAD, NSCLC | FDA guidelines |
| ALK | 5-UTRSNV | Different Alteration | Ceritinib (ALK inhibitor) | Responsive | IM, COREAD | Case report |
| ALK | 5-UTRSNV | Different Alteration | Crizotinib (ALK inhibitor) | Responsive | LUAD, NSCLC | FDA guidelines |
| ALK | 5-UTRSNV | Different Alteration | Brigatinib (Pan-TK inhibitor) | Responsive | NSCLC | FDA guidelines |
| ALK | 5-UTRSNV | Different Alteration | Entrictinib (Pan-TK inhibitor) | Responsive | COREAD | Case report |
| ALK | 5-UTRSNV | Different Alteration | Alectinib (ALK inhibitor) | Responsive | LUAD | FDA guidelines |
| ALK | 5-UTRSNV | Different Alteration | HSP90 inhibitor (HSP90 inhibitor) | Responsive | LUAD | Early trials |
| ALK | 5-UTRSNV | Different Alteration | ALK inhibitor;IGF1R inhibitor | Responsive | LUAD | Pre-clinical |
| ALK | 5-UTRSNV | Different Alteration | ALK inhibitor;MEK inhibitor | Responsive | LUAD | Pre-clinical |
| ALK | 5-UTRSNV | Different Alteration | novel ALK inhibitor | Responsive | LUAD | Early trials |
| ALK | 5-UTRSNV | Different Alteration | Ceritinib (ALK inhibitor) | Responsive | HEMATO | Early trials |
| ALK | 5-UTRSNV | Different Alteration | ALK inhibitor | Responsive | COREAD | Case report |
| ALK | 5-UTRSNV | Different Alteration | Alectinib (ALK inhibitor) | Responsive | NSCLC | FDA guidelines |
| ALK | 5-UTRSNV | Different Alteration | MEK inhibitor (ALK inhibitor;MEK inhibitor) | Responsive | LUAD | Pre-clinical |
| ALK | 5-UTRSNV), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_varian | Different Alteration | ALK inhibitor;SRC inhibitor | Responsive | LUAD | Pre-clinical |
| ALK | 5-UTRSNV), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_varian | Different Alteration | Crizotinib (ALK inhibitor) | Responsive | GB, LY | Early trials |
| ALK | 5-UTRSNV), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_varian | Different Alteration | Lorlatinib (ALK&ROS1 inhibitor) | Responsive | NSCLC | Early trials |
| ALK | 5-UTRSNV), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_varian | Different Alteration | Crizotinib (ALK inhibitor) | Responsive | THCA, IM | Case report |
| ALK | 5-UTRSNV), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_varian | Different Alteration | HSP90 inhibitor | Responsive | LUAD | Early trials |
| ALK | 5-UTRSNV), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_varian | Different Alteration | Ceritinib (ALK inhibitor) | Responsive | LUAD, NSCLC | FDA guidelines |
| ALK | 5-UTRSNV), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_varian | Different Alteration | Ceritinib (ALK inhibitor) | Responsive | IM, COREAD | Case report |
| ALK | 5-UTRSNV), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_varian | Different Alteration | Crizotinib (ALK inhibitor) | Responsive | LUAD, NSCLC | FDA guidelines |
| ALK | 5-UTRSNV), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_varian | Different Alteration | Brigatinib (Pan-TK inhibitor) | Responsive | NSCLC | FDA guidelines |
| ALK | 5-UTRSNV), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_varian | Different Alteration | Entrictinib (Pan-TK inhibitor) | Responsive | COREAD | Case report |
| ALK | 5-UTRSNV), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_varian | Different Alteration | Alectinib (ALK inhibitor) | Responsive | LUAD | FDA guidelines |
| ALK | 5-UTRSNV), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_varian | Different Alteration | HSP90 inhibitor (HSP90 inhibitor) | Responsive | LUAD | Early trials |
| ALK | 5-UTRSNV), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_varian | Different Alteration | ALK inhibitor;IGF1R inhibitor | Responsive | LUAD | Pre-clinical |
| ALK | 5-UTRSNV), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_varian | Different Alteration | ALK inhibitor;MEK inhibitor | Responsive | LUAD | Pre-clinical |
| ALK | 5-UTRSNV), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_varian | Different Alteration | novel ALK inhibitor | Responsive | LUAD | Early trials |
| ALK | 5-UTRSNV), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_varian | Different Alteration | Ceritinib (ALK inhibitor) | Responsive | HEMATO | Early trials |
| ALK | 5-UTRSNV), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_varian | Different Alteration | ALK inhibitor | Responsive | COREAD | Case report |
| ALK | 5-UTRSNV), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_varian | Different Alteration | Alectinib (ALK inhibitor) | Responsive | NSCLC | FDA guidelines |
| ALK | 5-UTRSNV), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_varian | Different Alteration | MEK inhibitor (ALK inhibitor;MEK inhibitor) | Responsive | LUAD | Pre-clinical |
| ALK | intron_variant), ALK MUT* (intron_variant), ALK MUT* (G574R | Different Alteration | ALK inhibitor;SRC inhibitor | Responsive | LUAD | Pre-clinical |
| ALK | intron_variant), ALK MUT* (intron_variant), ALK MUT* (G574R | Different Alteration | Crizotinib (ALK inhibitor) | Responsive | GB, LY | Early trials |
| ALK | intron_variant), ALK MUT* (intron_variant), ALK MUT* (G574R | Different Alteration | Lorlatinib (ALK&ROS1 inhibitor) | Responsive | NSCLC | Early trials |
| ALK | intron_variant), ALK MUT* (intron_variant), ALK MUT* (G574R | Different Alteration | Crizotinib (ALK inhibitor) | Responsive | THCA, IM | Case report |
| ALK | intron_variant), ALK MUT* (intron_variant), ALK MUT* (G574R | Different Alteration | HSP90 inhibitor | Responsive | LUAD | Early trials |
| ALK | intron_variant), ALK MUT* (intron_variant), ALK MUT* (G574R | Different Alteration | Ceritinib (ALK inhibitor) | Responsive | LUAD, NSCLC | FDA guidelines |
| ALK | intron_variant), ALK MUT* (intron_variant), ALK MUT* (G574R | Different Alteration | Ceritinib (ALK inhibitor) | Responsive | IM, COREAD | Case report |
| ALK | intron_variant), ALK MUT* (intron_variant), ALK MUT* (G574R | Different Alteration | Crizotinib (ALK inhibitor) | Responsive | LUAD, NSCLC | FDA guidelines |
| ALK | intron_variant), ALK MUT* (intron_variant), ALK MUT* (G574R | Different Alteration | Brigatinib (Pan-TK inhibitor) | Responsive | NSCLC | FDA guidelines |
| ALK | intron_variant), ALK MUT* (intron_variant), ALK MUT* (G574R | Different Alteration | Entrictinib (Pan-TK inhibitor) | Responsive | COREAD | Case report |
| ALK | intron_variant), ALK MUT* (intron_variant), ALK MUT* (G574R | Different Alteration | Alectinib (ALK inhibitor) | Responsive | LUAD | FDA guidelines |
| ALK | intron_variant), ALK MUT* (intron_variant), ALK MUT* (G574R | Different Alteration | HSP90 inhibitor (HSP90 inhibitor) | Responsive | LUAD | Early trials |
| ALK | intron_variant), ALK MUT* (intron_variant), ALK MUT* (G574R | Different Alteration | ALK inhibitor;IGF1R inhibitor | Responsive | LUAD | Pre-clinical |
| ALK | intron_variant), ALK MUT* (intron_variant), ALK MUT* (G574R | Different Alteration | ALK inhibitor;MEK inhibitor | Responsive | LUAD | Pre-clinical |
| ALK | intron_variant), ALK MUT* (intron_variant), ALK MUT* (G574R | Different Alteration | novel ALK inhibitor | Responsive | LUAD | Early trials |
| ALK | intron_variant), ALK MUT* (intron_variant), ALK MUT* (G574R | Different Alteration | Ceritinib (ALK inhibitor) | Responsive | HEMATO | Early trials |
| ALK | intron_variant), ALK MUT* (intron_variant), ALK MUT* (G574R | Different Alteration | ALK inhibitor | Responsive | COREAD | Case report |
| ALK | intron_variant), ALK MUT* (intron_variant), ALK MUT* (G574R | Different Alteration | Alectinib (ALK inhibitor) | Responsive | NSCLC | FDA guidelines |
| ALK | intron_variant), ALK MUT* (intron_variant), ALK MUT* (G574R | Different Alteration | MEK inhibitor (ALK inhibitor;MEK inhibitor) | Responsive | LUAD | Pre-clinical |
| APC | R1450* | Complete Match | Tankyrase inhibitor | Responsive | COREAD | Pre-clinical |
| APC | R1858* | Complete Match | Tankyrase inhibitor | Responsive | COREAD | Pre-clinical |
| ARID1A | Q542* | Complete Match | EZH2 inhibitor | Responsive | OV | Pre-clinical |
| ARID1A | Q542* | Complete Match | PD1 inhibitor | Responsive | OV | Pre-clinical |
| ARID1A | Q542* | Complete Match | PARP inhibitor | Responsive | CANCER | Pre-clinical |
| ARID1A | Q542* | Complete Match | ATR inhibitor | Responsive | CANCER | Pre-clinical |
| ARID1A | Q1473* | Complete Match | EZH2 inhibitor | Responsive | OV | Pre-clinical |
| ARID1A | Q1473* | Complete Match | PD1 inhibitor | Responsive | OV | Pre-clinical |
| ARID1A | Q1473* | Complete Match | PARP inhibitor | Responsive | CANCER | Pre-clinical |
| ARID1A | Q1473* | Complete Match | ATR inhibitor | Responsive | CANCER | Pre-clinical |
| ARID1A | Q766* | Complete Match | EZH2 inhibitor | Responsive | OV | Pre-clinical |
| ARID1A | Q766* | Complete Match | PD1 inhibitor | Responsive | OV | Pre-clinical |
| ARID1A | Q766* | Complete Match | PARP inhibitor | Responsive | CANCER | Pre-clinical |
| ARID1A | Q766* | Complete Match | ATR inhibitor | Responsive | CANCER | Pre-clinical |
| ARID1A | R1989* | Complete Match | EZH2 inhibitor | Responsive | OV | Pre-clinical |
| ARID1A | R1989* | Complete Match | PD1 inhibitor | Responsive | OV | Pre-clinical |
| ARID1A | R1989* | Complete Match | PARP inhibitor | Responsive | CANCER | Pre-clinical |
| ARID1A | R1989* | Complete Match | ATR inhibitor | Responsive | CANCER | Pre-clinical |
| ARID1A | Q529* | Complete Match | EZH2 inhibitor | Responsive | OV | Pre-clinical |
| ARID1A | Q529* | Complete Match | PD1 inhibitor | Responsive | OV | Pre-clinical |
| ARID1A | Q529* | Complete Match | PARP inhibitor | Responsive | CANCER | Pre-clinical |
| ARID1A | Q529* | Complete Match | ATR inhibitor | Responsive | CANCER | Pre-clinical |
| ARID1A | splice_acceptor_variant | Complete Match | EZH2 inhibitor | Responsive | OV | Pre-clinical |
| ARID1A | splice_acceptor_variant | Complete Match | PD1 inhibitor | Responsive | OV | Pre-clinical |
| ARID1A | splice_acceptor_variant | Complete Match | PARP inhibitor | Responsive | CANCER | Pre-clinical |
| ARID1A | splice_acceptor_variant | Complete Match | ATR inhibitor | Responsive | CANCER | Pre-clinical |
| ARID1A | Q563* | Complete Match | EZH2 inhibitor | Responsive | OV | Pre-clinical |
| ARID1A | Q563* | Complete Match | PD1 inhibitor | Responsive | OV | Pre-clinical |
| ARID1A | Q563* | Complete Match | PARP inhibitor | Responsive | CANCER | Pre-clinical |
| ARID1A | Q563* | Complete Match | ATR inhibitor | Responsive | CANCER | Pre-clinical |
| ARID1A | W1973* | Complete Match | EZH2 inhibitor | Responsive | OV | Pre-clinical |
| ARID1A | W1973* | Complete Match | PD1 inhibitor | Responsive | OV | Pre-clinical |
| ARID1A | W1973* | Complete Match | PARP inhibitor | Responsive | CANCER | Pre-clinical |
| ARID1A | W1973* | Complete Match | ATR inhibitor | Responsive | CANCER | Pre-clinical |
| ARID1A | G655* | Complete Match | EZH2 inhibitor | Responsive | OV | Pre-clinical |
| ARID1A | G655* | Complete Match | PD1 inhibitor | Responsive | OV | Pre-clinical |
| ARID1A | G655* | Complete Match | PARP inhibitor | Responsive | CANCER | Pre-clinical |
| ARID1A | G655* | Complete Match | ATR inhibitor | Responsive | CANCER | Pre-clinical |
| ARID1A | Q1095* | Complete Match | EZH2 inhibitor | Responsive | OV | Pre-clinical |
| ARID1A | Q1095* | Complete Match | PD1 inhibitor | Responsive | OV | Pre-clinical |
| ARID1A | Q1095* | Complete Match | PARP inhibitor | Responsive | CANCER | Pre-clinical |
| ARID1A | Q1095* | Complete Match | ATR inhibitor | Responsive | CANCER | Pre-clinical |
| ATM | E2784* | Different Mutation | AZD6738 (ATR inhibitor) | Responsive | BCL | Early trials |
| ATM | E2784* | Complete Match | Cisplatin (Chemotherapy) | Responsive | BLCA | Early trials |
| ATM | E2784* | Complete Match | Olaparib (PARP inhibitor) | Responsive | ST, PRAD | Early trials |
| ATM | E2784* | Complete Match | PARP inhibitor | Responsive | ST | Early trials |
| ATM | E2784* | Complete Match | ATR inhibitor | Responsive | COREAD | Case report |
| ATM | E2784* | Complete Match | DNA-PKc inhibitor | Responsive | LY | Pre-clinical |
| ATM | E2784* | Complete Match | Temozolomide (Chemotherapy) | Responsive | G | Pre-clinical |
| ATM | E2784* | Complete Match | PD1/PDL1 inhibitor | Responsive | UTH | Early trials |
| ATM | E2784* | Different Alteration | PD1/PDL1 inhibitor | Responsive | UTH | Early trials |
| ATM | E2784* | Different Alteration | DNA-PKc inhibitor | Responsive | LY | Pre-clinical |
| ATM | E2784* | Different Alteration | PARP inhibitor | Responsive | ST | Early trials |
| ATM | E2784* | Different Alteration | ATR inhibitor | Responsive | COREAD | Case report |
| ATM | E2784* | Different Alteration | Cisplatin (Chemotherapy) | Responsive | BLCA | Early trials |
| ATM | R1466* | Different Mutation | AZD6738 (ATR inhibitor) | Responsive | BCL | Early trials |
| ATM | R1466* | Complete Match | Cisplatin (Chemotherapy) | Responsive | BLCA | Early trials |
| ATM | R1466* | Complete Match | Olaparib (PARP inhibitor) | Responsive | ST, PRAD | Early trials |
| ATM | R1466* | Complete Match | PARP inhibitor | Responsive | ST | Early trials |
| ATM | R1466* | Complete Match | ATR inhibitor | Responsive | COREAD | Case report |
| ATM | R1466* | Complete Match | DNA-PKc inhibitor | Responsive | LY | Pre-clinical |
| ATM | R1466* | Complete Match | Temozolomide (Chemotherapy) | Responsive | G | Pre-clinical |
| ATM | R1466* | Complete Match | PD1/PDL1 inhibitor | Responsive | UTH | Early trials |
| ATM | R1466* | Different Alteration | PD1/PDL1 inhibitor | Responsive | UTH | Early trials |
| ATM | R1466* | Different Alteration | DNA-PKc inhibitor | Responsive | LY | Pre-clinical |
| ATM | R1466* | Different Alteration | PARP inhibitor | Responsive | ST | Early trials |
| ATM | R1466* | Different Alteration | ATR inhibitor | Responsive | COREAD | Case report |
| ATM | R1466* | Different Alteration | Cisplatin (Chemotherapy) | Responsive | BLCA | Early trials |
| ATM | splice_acceptor_variant | Different Mutation | AZD6738 (ATR inhibitor) | Responsive | BCL | Early trials |
| ATM | splice_acceptor_variant | Complete Match | Cisplatin (Chemotherapy) | Responsive | BLCA | Early trials |
| ATM | splice_acceptor_variant | Complete Match | Olaparib (PARP inhibitor) | Responsive | ST, PRAD | Early trials |
| ATM | splice_acceptor_variant | Complete Match | PARP inhibitor | Responsive | ST | Early trials |
| ATM | splice_acceptor_variant | Complete Match | ATR inhibitor | Responsive | COREAD | Case report |
| ATM | splice_acceptor_variant | Complete Match | DNA-PKc inhibitor | Responsive | LY | Pre-clinical |
| ATM | splice_acceptor_variant | Complete Match | Temozolomide (Chemotherapy) | Responsive | G | Pre-clinical |
| ATM | splice_acceptor_variant | Complete Match | PD1/PDL1 inhibitor | Responsive | UTH | Early trials |
| ATM | splice_acceptor_variant | Different Alteration | PD1/PDL1 inhibitor | Responsive | UTH | Early trials |
| ATM | splice_acceptor_variant | Different Alteration | DNA-PKc inhibitor | Responsive | LY | Pre-clinical |
| ATM | splice_acceptor_variant | Different Alteration | PARP inhibitor | Responsive | ST | Early trials |
| ATM | splice_acceptor_variant | Different Alteration | ATR inhibitor | Responsive | COREAD | Case report |
| ATM | splice_acceptor_variant | Different Alteration | Cisplatin (Chemotherapy) | Responsive | BLCA | Early trials |
| BCR | intron_variant | Different Alteration | Ponatinib (BCR-ABL inhibitor & Pan-TK inhibitor) | Responsive | CML, ALL | FDA guidelines |
| BCR | intron_variant | Different Alteration | Imatinib (BCR-ABL inhibitor & KIT inhibitor) | Responsive | ALL, CML | FDA guidelines |
| BCR | intron_variant | Different Alteration | Nilotinib (BCR-ABL inhibitor) | Responsive | CML | FDA guidelines |
| BCR | intron_variant | Different Alteration | Bosutinib (BCR-ABL inhibitor) | Responsive | CML | FDA guidelines |
| BCR | intron_variant | Different Alteration | Dasatinib;Venetoclax (BCR-ABL inhibitor;BCL2 inhibitor) | Responsive | ALL | Pre-clinical |
| BCR | intron_variant | Different Alteration | Dasatinib (BCR-ABL inhibitor) | Responsive | ALL, CML | FDA guidelines |
| BCR | intron_variant), BCR MUT* (intron_variant | Different Alteration | Ponatinib (BCR-ABL inhibitor & Pan-TK inhibitor) | Responsive | CML, ALL | FDA guidelines |
| BCR | intron_variant), BCR MUT* (intron_variant | Different Alteration | Imatinib (BCR-ABL inhibitor & KIT inhibitor) | Responsive | ALL, CML | FDA guidelines |
| BCR | intron_variant), BCR MUT* (intron_variant | Different Alteration | Nilotinib (BCR-ABL inhibitor) | Responsive | CML | FDA guidelines |
| BCR | intron_variant), BCR MUT* (intron_variant | Different Alteration | Bosutinib (BCR-ABL inhibitor) | Responsive | CML | FDA guidelines |
| BCR | intron_variant), BCR MUT* (intron_variant | Different Alteration | Dasatinib;Venetoclax (BCR-ABL inhibitor;BCL2 inhibitor) | Responsive | ALL | Pre-clinical |
| BCR | intron_variant), BCR MUT* (intron_variant | Different Alteration | Dasatinib (BCR-ABL inhibitor) | Responsive | ALL, CML | FDA guidelines |
| BCR | IntronicBlockSubstitution | Different Alteration | Ponatinib (BCR-ABL inhibitor & Pan-TK inhibitor) | Responsive | CML, ALL | FDA guidelines |
| BCR | IntronicBlockSubstitution | Different Alteration | Imatinib (BCR-ABL inhibitor & KIT inhibitor) | Responsive | ALL, CML | FDA guidelines |
| BCR | IntronicBlockSubstitution | Different Alteration | Nilotinib (BCR-ABL inhibitor) | Responsive | CML | FDA guidelines |
| BCR | IntronicBlockSubstitution | Different Alteration | Bosutinib (BCR-ABL inhibitor) | Responsive | CML | FDA guidelines |
| BCR | IntronicBlockSubstitution | Different Alteration | Dasatinib;Venetoclax (BCR-ABL inhibitor;BCL2 inhibitor) | Responsive | ALL | Pre-clinical |
| BCR | IntronicBlockSubstitution | Different Alteration | Dasatinib (BCR-ABL inhibitor) | Responsive | ALL, CML | FDA guidelines |
| BCR | intron_variant), BCR MUT* (intron_variant), BCR MUT* (intron_variant), BCR MUT* (intron_variant), BCR MUT* (intron_variant), BCR MUT* (intron_variant), BCR MUT* (intron_variant), BCR MUT* (intron_variant | Different Alteration | Ponatinib (BCR-ABL inhibitor & Pan-TK inhibitor) | Responsive | CML, ALL | FDA guidelines |
| BCR | intron_variant), BCR MUT* (intron_variant), BCR MUT* (intron_variant), BCR MUT* (intron_variant), BCR MUT* (intron_variant), BCR MUT* (intron_variant), BCR MUT* (intron_variant), BCR MUT* (intron_variant | Different Alteration | Imatinib (BCR-ABL inhibitor & KIT inhibitor) | Responsive | ALL, CML | FDA guidelines |
| BCR | intron_variant), BCR MUT* (intron_variant), BCR MUT* (intron_variant), BCR MUT* (intron_variant), BCR MUT* (intron_variant), BCR MUT* (intron_variant), BCR MUT* (intron_variant), BCR MUT* (intron_variant | Different Alteration | Nilotinib (BCR-ABL inhibitor) | Responsive | CML | FDA guidelines |
| BCR | intron_variant), BCR MUT* (intron_variant), BCR MUT* (intron_variant), BCR MUT* (intron_variant), BCR MUT* (intron_variant), BCR MUT* (intron_variant), BCR MUT* (intron_variant), BCR MUT* (intron_variant | Different Alteration | Bosutinib (BCR-ABL inhibitor) | Responsive | CML | FDA guidelines |
| BCR | intron_variant), BCR MUT* (intron_variant), BCR MUT* (intron_variant), BCR MUT* (intron_variant), BCR MUT* (intron_variant), BCR MUT* (intron_variant), BCR MUT* (intron_variant), BCR MUT* (intron_variant | Different Alteration | Dasatinib;Venetoclax (BCR-ABL inhibitor;BCL2 inhibitor) | Responsive | ALL | Pre-clinical |
| BCR | intron_variant), BCR MUT* (intron_variant), BCR MUT* (intron_variant), BCR MUT* (intron_variant), BCR MUT* (intron_variant), BCR MUT* (intron_variant), BCR MUT* (intron_variant), BCR MUT* (intron_variant | Different Alteration | Dasatinib (BCR-ABL inhibitor) | Responsive | ALL, CML | FDA guidelines |
| BCR | intron_variant), BCR MUT* (intron_variant), BCR MUT* (intron_variant), BCR MUT* (intron_variant), BCR MUT* (intron_variant | Different Alteration | Ponatinib (BCR-ABL inhibitor & Pan-TK inhibitor) | Responsive | CML, ALL | FDA guidelines |
| BCR | intron_variant), BCR MUT* (intron_variant), BCR MUT* (intron_variant), BCR MUT* (intron_variant), BCR MUT* (intron_variant | Different Alteration | Imatinib (BCR-ABL inhibitor & KIT inhibitor) | Responsive | ALL, CML | FDA guidelines |
| BCR | intron_variant), BCR MUT* (intron_variant), BCR MUT* (intron_variant), BCR MUT* (intron_variant), BCR MUT* (intron_variant | Different Alteration | Nilotinib (BCR-ABL inhibitor) | Responsive | CML | FDA guidelines |
| BCR | intron_variant), BCR MUT* (intron_variant), BCR MUT* (intron_variant), BCR MUT* (intron_variant), BCR MUT* (intron_variant | Different Alteration | Bosutinib (BCR-ABL inhibitor) | Responsive | CML | FDA guidelines |
| BCR | intron_variant), BCR MUT* (intron_variant), BCR MUT* (intron_variant), BCR MUT* (intron_variant), BCR MUT* (intron_variant | Different Alteration | Dasatinib;Venetoclax (BCR-ABL inhibitor;BCL2 inhibitor) | Responsive | ALL | Pre-clinical |
| BCR | intron_variant), BCR MUT* (intron_variant), BCR MUT* (intron_variant), BCR MUT* (intron_variant), BCR MUT* (intron_variant | Different Alteration | Dasatinib (BCR-ABL inhibitor) | Responsive | ALL, CML | FDA guidelines |
| BCR | T1205T), BCR MUT* (A1204G | Different Alteration | Ponatinib (BCR-ABL inhibitor & Pan-TK inhibitor) | Responsive | CML, ALL | FDA guidelines |
| BCR | T1205T), BCR MUT* (A1204G | Different Alteration | Imatinib (BCR-ABL inhibitor & KIT inhibitor) | Responsive | ALL, CML | FDA guidelines |
| BCR | T1205T), BCR MUT* (A1204G | Different Alteration | Nilotinib (BCR-ABL inhibitor) | Responsive | CML | FDA guidelines |
| BCR | T1205T), BCR MUT* (A1204G | Different Alteration | Bosutinib (BCR-ABL inhibitor) | Responsive | CML | FDA guidelines |
| BCR | T1205T), BCR MUT* (A1204G | Different Alteration | Dasatinib;Venetoclax (BCR-ABL inhibitor;BCL2 inhibitor) | Responsive | ALL | Pre-clinical |
| BCR | T1205T), BCR MUT* (A1204G | Different Alteration | Dasatinib (BCR-ABL inhibitor) | Responsive | ALL, CML | FDA guidelines |
| BCR | R695* | Different Alteration | Ponatinib (BCR-ABL inhibitor & Pan-TK inhibitor) | Responsive | CML, ALL | FDA guidelines |
| BCR | R695* | Different Alteration | Imatinib (BCR-ABL inhibitor & KIT inhibitor) | Responsive | ALL, CML | FDA guidelines |
| BCR | R695* | Different Alteration | Nilotinib (BCR-ABL inhibitor) | Responsive | CML | FDA guidelines |
| BCR | R695* | Different Alteration | Bosutinib (BCR-ABL inhibitor) | Responsive | CML | FDA guidelines |
| BCR | R695* | Different Alteration | Dasatinib;Venetoclax (BCR-ABL inhibitor;BCL2 inhibitor) | Responsive | ALL | Pre-clinical |
| BCR | R695* | Different Alteration | Dasatinib (BCR-ABL inhibitor) | Responsive | ALL, CML | FDA guidelines |
| BCR | E1077E), BCR MUT* (intron_variant | Different Alteration | Ponatinib (BCR-ABL inhibitor & Pan-TK inhibitor) | Responsive | CML, ALL | FDA guidelines |
| BCR | E1077E), BCR MUT* (intron_variant | Different Alteration | Imatinib (BCR-ABL inhibitor & KIT inhibitor) | Responsive | ALL, CML | FDA guidelines |
| BCR | E1077E), BCR MUT* (intron_variant | Different Alteration | Nilotinib (BCR-ABL inhibitor) | Responsive | CML | FDA guidelines |
| BCR | E1077E), BCR MUT* (intron_variant | Different Alteration | Bosutinib (BCR-ABL inhibitor) | Responsive | CML | FDA guidelines |
| BCR | E1077E), BCR MUT* (intron_variant | Different Alteration | Dasatinib;Venetoclax (BCR-ABL inhibitor;BCL2 inhibitor) | Responsive | ALL | Pre-clinical |
| BCR | E1077E), BCR MUT* (intron_variant | Different Alteration | Dasatinib (BCR-ABL inhibitor) | Responsive | ALL, CML | FDA guidelines |
| BCR | 3-UTRSNV), BCR MUT* (intron_variant | Different Alteration | Ponatinib (BCR-ABL inhibitor & Pan-TK inhibitor) | Responsive | CML, ALL | FDA guidelines |
| BCR | 3-UTRSNV), BCR MUT* (intron_variant | Different Alteration | Imatinib (BCR-ABL inhibitor & KIT inhibitor) | Responsive | ALL, CML | FDA guidelines |
| BCR | 3-UTRSNV), BCR MUT* (intron_variant | Different Alteration | Nilotinib (BCR-ABL inhibitor) | Responsive | CML | FDA guidelines |
| BCR | 3-UTRSNV), BCR MUT* (intron_variant | Different Alteration | Bosutinib (BCR-ABL inhibitor) | Responsive | CML | FDA guidelines |
| BCR | 3-UTRSNV), BCR MUT* (intron_variant | Different Alteration | Dasatinib;Venetoclax (BCR-ABL inhibitor;BCL2 inhibitor) | Responsive | ALL | Pre-clinical |
| BCR | 3-UTRSNV), BCR MUT* (intron_variant | Different Alteration | Dasatinib (BCR-ABL inhibitor) | Responsive | ALL, CML | FDA guidelines |
| BCR | intron_variant), BCR MUT* (intron_variant), BCR MUT* (intron_variant | Different Alteration | Ponatinib (BCR-ABL inhibitor & Pan-TK inhibitor) | Responsive | CML, ALL | FDA guidelines |
| BCR | intron_variant), BCR MUT* (intron_variant), BCR MUT* (intron_variant | Different Alteration | Imatinib (BCR-ABL inhibitor & KIT inhibitor) | Responsive | ALL, CML | FDA guidelines |
| BCR | intron_variant), BCR MUT* (intron_variant), BCR MUT* (intron_variant | Different Alteration | Nilotinib (BCR-ABL inhibitor) | Responsive | CML | FDA guidelines |
| BCR | intron_variant), BCR MUT* (intron_variant), BCR MUT* (intron_variant | Different Alteration | Bosutinib (BCR-ABL inhibitor) | Responsive | CML | FDA guidelines |
| BCR | intron_variant), BCR MUT* (intron_variant), BCR MUT* (intron_variant | Different Alteration | Dasatinib;Venetoclax (BCR-ABL inhibitor;BCL2 inhibitor) | Responsive | ALL | Pre-clinical |
| BCR | intron_variant), BCR MUT* (intron_variant), BCR MUT* (intron_variant | Different Alteration | Dasatinib (BCR-ABL inhibitor) | Responsive | ALL, CML | FDA guidelines |
| BCR | V398V | Different Alteration | Ponatinib (BCR-ABL inhibitor & Pan-TK inhibitor) | Responsive | CML, ALL | FDA guidelines |
| BCR | V398V | Different Alteration | Imatinib (BCR-ABL inhibitor & KIT inhibitor) | Responsive | ALL, CML | FDA guidelines |
| BCR | V398V | Different Alteration | Nilotinib (BCR-ABL inhibitor) | Responsive | CML | FDA guidelines |
| BCR | V398V | Different Alteration | Bosutinib (BCR-ABL inhibitor) | Responsive | CML | FDA guidelines |
| BCR | V398V | Different Alteration | Dasatinib;Venetoclax (BCR-ABL inhibitor;BCL2 inhibitor) | Responsive | ALL | Pre-clinical |
| BCR | V398V | Different Alteration | Dasatinib (BCR-ABL inhibitor) | Responsive | ALL, CML | FDA guidelines |
| BRAF | intron_variant | Different Alteration | Sorafenib (Pan-TK inhibitor) | Responsive | CM, LUAD, PRAD | Pre-clinical |
| BRAF | intron_variant | Different Alteration | MEK inhibitor | Responsive | LUAD, CM, PRAD | Pre-clinical |
| BRAF | intron_variant | Different Alteration | Selumetinib (MEK inhibitor) | Responsive | OV | Case report |
| BRAF | intron_variant | Different Alteration | BRAF inhibitor;MEK inhibitor | Responsive | LUAD | Case report |
| BRAF | intron_variant), BRAF MUT* (intron_variant | Different Alteration | Sorafenib (Pan-TK inhibitor) | Responsive | CM, LUAD, PRAD | Pre-clinical |
| BRAF | intron_variant), BRAF MUT* (intron_variant | Different Alteration | MEK inhibitor | Responsive | LUAD, CM, PRAD | Pre-clinical |
| BRAF | intron_variant), BRAF MUT* (intron_variant | Different Alteration | Selumetinib (MEK inhibitor) | Responsive | OV | Case report |
| BRAF | intron_variant), BRAF MUT* (intron_variant | Different Alteration | BRAF inhibitor;MEK inhibitor | Responsive | LUAD | Case report |
| BRAF | intron_variant), BRAF MUT* (intron_variant), BRAF MUT* (intron_variant | Different Alteration | Sorafenib (Pan-TK inhibitor) | Responsive | CM, LUAD, PRAD | Pre-clinical |
| BRAF | intron_variant), BRAF MUT* (intron_variant), BRAF MUT* (intron_variant | Different Alteration | MEK inhibitor | Responsive | LUAD, CM, PRAD | Pre-clinical |
| BRAF | intron_variant), BRAF MUT* (intron_variant), BRAF MUT* (intron_variant | Different Alteration | Selumetinib (MEK inhibitor) | Responsive | OV | Case report |
| BRAF | intron_variant), BRAF MUT* (intron_variant), BRAF MUT* (intron_variant | Different Alteration | BRAF inhibitor;MEK inhibitor | Responsive | LUAD | Case report |
| BRAF | intron_variant), BRAF MUT* (intron_variant), BRAF MUT* (intron_variant), BRAF MUT* (intron_variant), BRAF MUT* (intron_variant), BRAF MUT* (intron_variant), BRAF MUT* (S727G | Different Alteration | Sorafenib (Pan-TK inhibitor) | Responsive | CM, LUAD, PRAD | Pre-clinical |
| BRAF | intron_variant), BRAF MUT* (intron_variant), BRAF MUT* (intron_variant), BRAF MUT* (intron_variant), BRAF MUT* (intron_variant), BRAF MUT* (intron_variant), BRAF MUT* (S727G | Different Alteration | MEK inhibitor | Responsive | LUAD, CM, PRAD | Pre-clinical |
| BRAF | intron_variant), BRAF MUT* (intron_variant), BRAF MUT* (intron_variant), BRAF MUT* (intron_variant), BRAF MUT* (intron_variant), BRAF MUT* (intron_variant), BRAF MUT* (S727G | Different Alteration | Selumetinib (MEK inhibitor) | Responsive | OV | Case report |
| BRAF | intron_variant), BRAF MUT* (intron_variant), BRAF MUT* (intron_variant), BRAF MUT* (intron_variant), BRAF MUT* (intron_variant), BRAF MUT* (intron_variant), BRAF MUT* (S727G | Different Alteration | BRAF inhibitor;MEK inhibitor | Responsive | LUAD | Case report |
| BRAF | intron_variant), BRAF MUT* (intron_variant), BRAF MUT* (intron_variant), BRAF MUT* (intron_variant), BRAF MUT* (intron_variant), BRAF MUT* (intron_variant | Different Alteration | Sorafenib (Pan-TK inhibitor) | Responsive | CM, LUAD, PRAD | Pre-clinical |
| BRAF | intron_variant), BRAF MUT* (intron_variant), BRAF MUT* (intron_variant), BRAF MUT* (intron_variant), BRAF MUT* (intron_variant), BRAF MUT* (intron_variant | Different Alteration | MEK inhibitor | Responsive | LUAD, CM, PRAD | Pre-clinical |
| BRAF | intron_variant), BRAF MUT* (intron_variant), BRAF MUT* (intron_variant), BRAF MUT* (intron_variant), BRAF MUT* (intron_variant), BRAF MUT* (intron_variant | Different Alteration | Selumetinib (MEK inhibitor) | Responsive | OV | Case report |
| BRAF | intron_variant), BRAF MUT* (intron_variant), BRAF MUT* (intron_variant), BRAF MUT* (intron_variant), BRAF MUT* (intron_variant), BRAF MUT* (intron_variant | Different Alteration | BRAF inhibitor;MEK inhibitor | Responsive | LUAD | Case report |
| BRAF | D594G | Different Mutation | Dasatinib (BCR-ABL inhibitor) | Responsive | LUAD | Pre-clinical |
| BRAF | D594G | Different Mutation | Vemurafenib (BRAF inhibitor) | Responsive | HISEC, HISLC, NSCLC | NCCN guidelines |
| BRAF | D594G | Different Mutation | Vemurafenib (BRAF inhibitor) | Responsive | CM | NCCN guidelines |
| BRAF | D594G | Different Mutation | Vemurafenib;Cobimetinib (BRAF inhibitor;MEK inhibitor) | Responsive | CM | FDA guidelines |
| BRAF | D594G | Different Mutation | Trametinib (MEK inhibitor) | Responsive | CM | FDA guidelines |
| BRAF | D594G | Different Mutation | Dabrafenib;Trametinib (BRAF inhibitor;MEK inhibitor) | Responsive | CM | FDA guidelines |
| BRAF | D594G | Different Mutation | Pan-RAF inhibitor | Responsive | CANCER | Pre-clinical |
| BRAF | D594G | Different Mutation | Vemurafenib (BRAF inhibitor) | Resistant | CANCER | Pre-clinical |
| BRAF | D594G | Different Mutation | ERK inhibitor | Responsive | HNSC, BT | Case report |
| BRAF | D594G | Different Mutation | EGFR TK inhibitor | Resistant | LUAD | Case report |
| BRAF | D594G | Different Mutation | MEK inhibitor | Responsive | CM | Case report |
| BRAF | D594G | Different Mutation | Dabrafenib (BRAF inhibitor) | Responsive | CM | Early trials |
| BRAF | D594G | Different Mutation | BRAF inhibitor | Responsive | CM | Case report |
| BRAF | D594G | Complete Match | Sorafenib (Pan-TK inhibitor) | Responsive | CM | Pre-clinical |
| BRAF | D594G | Different Mutation | Vemurafenib (BRAF inhibitor) | No Responsive | COREAD | Early trials |
| BRAF | D594G | Different Mutation | MEK inhibitor (BRAF inhibitor;MEK inhibitor;anti-EGFR mAb inhibitor) | Responsive | COREAD | Early trials |
| BRAF | D594G | Different Mutation | Irinotecan;Vemurafenib;Cetuximab (TOPO1 inhibitor;BRAF inhibitor;EGFR mAb inhibitor) | Responsive | COREAD | NCCN guidelines |
| BRAF | D594G | Different Mutation | Panitumumab;Dabrafenib;BYL719 (EGFR mAb inhibitor;BRAF inhibitor;PI3K inhibitor) | Responsive | COREAD | Early trials |
| BRAF | D594G | Different Mutation | BRAF inhibitor;anti-EGFR mAb inhibitor +/- PI3K pathway inhibitor | Responsive | COREAD | Early trials |
| BRAF | D594G | Different Mutation | BRAF inhibitor;EGFR mAb inhibitor +/- PI3K inhibitor | Responsive | COREAD | Early trials |
| BRAF | D594G | Different Mutation | Dabrafenib;Trametinib (BRAF inhibitor;MEK inhibitor) | Responsive | COREAD | Early trials |
| BRAF | D594G | Different Mutation | Vemurafenib (BRAF inhibitor) | Responsive | MA | Early trials |
| BRAF | D594G | Different Mutation | BRAF inhibitor;MEK inhibitor | Responsive | TH | Early trials |
| BRAF | D594G | Different Mutation | BRAF inhibitor;HSP90 inhibitor | Responsive | CM | Pre-clinical |
| BRAF | D594G | Different Mutation | Panitumumab (EGFR mAb inhibitor) | Resistant | COREAD | Late trials |
| BRAF | D594G | Different Mutation | Panitumumab;Dabrafenib;Trametinib (EGFR mAb inhibitor;BRAF inhibitor;MEK inhibitor) | Responsive | COREAD | Early trials |
| BRAF | D594G | Different Mutation | Dabrafenib;Trametinib (BRAF inhibitor;MEK inhibitor) | Responsive | LUAD | FDA guidelines |
| BRAF | D594G | Different Mutation | Dabrafenib (BRAF inhibitor) | Responsive | NSCLC | NCCN guidelines |
| BRAF | D594G | Different Mutation | MEK inhibitor | Responsive | TH | Early trials |
| BRAF | D594G | Different Mutation | BRAF inhibitor;PI3K pathway inhibitor | Responsive | CM | Pre-clinical |
| BRAF | D594G | Different Mutation | Dabrafenib (BRAF inhibitor) | Responsive | LUAD, TH | Early trials |
| BRAF | D594G | Different Mutation | MEK inhibitor | Responsive | OV | Pre-clinical |
| BRAF | D594G | Different Mutation | Vemurafenib (BRAF inhibitor) | Responsive | CM | FDA guidelines |
| BRAF | D594G | Different Mutation | Vemurafenib (BRAF inhibitor) | Responsive | HCL, LUAD, MYMA | Case report |
| BRAF | D594G | Different Mutation | Pan-RAF inhibitor | Responsive | CM | Early trials |
| BRAF | D594G | Different Mutation | Cetuximab (EGFR mAb inhibitor) | Resistant | COREAD | Late trials |
| BRAF | D594G | Different Mutation | BRAF inhibitor | Responsive | G | Pre-clinical |
| BRAF | D594G | Different Mutation | BRAF inhibitor;CDK2/4 inhibitor | Responsive | CM | Pre-clinical |
| BRAF | D594G | Different Mutation | PLX4720 (BRAF inhibitor) | Responsive | MA | Pre-clinical |
| BRAF | D594G | Different Mutation | Vemurafenib (BRAF inhibitor) | Responsive | THCA | Early trials |
| BRAF | D594G | Different Mutation | Selumetinib (MEK inhibitor) | Responsive | PG | Early trials |
| BRAF | D594G | Different Mutation | ERK inhibitor | Responsive | LUAD | Early trials |
| BRAF | D594G | Different Mutation | ERK inhibitor | Responsive | CM | Pre-clinical |
| BRAF | D594G | Different Mutation | Dabrafenib;Trametinib (BRAF inhibitor;MEK inhibitor) | Responsive | NEU | Case report |
| BRAF | D594G | Different Mutation | BRAF inhibitor | Responsive | OV | Case report |
| BRAF | D594G | Different Mutation | Dabrafenib (BRAF inhibitor) | Responsive | GIST | Case report |
| BRAF | D594G | Different Mutation | Dabrafenib (BRAF inhibitor) | Responsive | CM | FDA guidelines |
| BRAF | D594G | Different Mutation | Dasatinib (BCR-ABL inhibitor) | Responsive | LUAD | Case report |
| BRAF | D594G), BRAF MUT* (intron_variant | Different Alteration | Sorafenib (Pan-TK inhibitor) | Responsive | CM, LUAD, PRAD | Pre-clinical |
| BRAF | D594G), BRAF MUT* (intron_variant | Different Alteration | MEK inhibitor | Responsive | LUAD, CM, PRAD | Pre-clinical |
| BRAF | D594G), BRAF MUT* (intron_variant | Different Alteration | Selumetinib (MEK inhibitor) | Responsive | OV | Case report |
| BRAF | D594G), BRAF MUT* (intron_variant | Different Alteration | BRAF inhibitor;MEK inhibitor | Responsive | LUAD | Case report |
| BRAF | P334L | Different Alteration | Sorafenib (Pan-TK inhibitor) | Responsive | CM, LUAD, PRAD | Pre-clinical |
| BRAF | P334L | Different Alteration | MEK inhibitor | Responsive | LUAD, CM, PRAD | Pre-clinical |
| BRAF | P334L | Different Alteration | Selumetinib (MEK inhibitor) | Responsive | OV | Case report |
| BRAF | P334L | Different Alteration | BRAF inhibitor;MEK inhibitor | Responsive | LUAD | Case report |
| BRAF | A305A | Different Alteration | Sorafenib (Pan-TK inhibitor) | Responsive | CM, LUAD, PRAD | Pre-clinical |
| BRAF | A305A | Different Alteration | MEK inhibitor | Responsive | LUAD, CM, PRAD | Pre-clinical |
| BRAF | A305A | Different Alteration | Selumetinib (MEK inhibitor) | Responsive | OV | Case report |
| BRAF | A305A | Different Alteration | BRAF inhibitor;MEK inhibitor | Responsive | LUAD | Case report |
| BRCA2 | splice_donor_variant | Complete Match | PD1 Ab inhibitor | Responsive | CM | Case report |
| BRCA2 | splice_donor_variant | Complete Match | Olaparib (PARP inhibitor) | Responsive | OV | FDA guidelines |
| BRCA2 | splice_donor_variant | Complete Match | Platinum Agent (Chemotherapy) | Responsive | OV | Late trials |
| BRCA2 | splice_donor_variant | Complete Match | Chemotherapy (PARP inhibitor;Chemotherapy) | Responsive | OV | Early trials |
| BRCA2 | splice_donor_variant | Complete Match | PARP inhibitor;Chemotherapy | Responsive | OV | Early trials |
| BRCA2 | splice_donor_variant | Complete Match | Platinum Agent (Chemotherapy) | Responsive | PA | Case report |
| BRCA2 | splice_donor_variant | Complete Match | Olaparib (PARP inhibitor) | Responsive | PRAD | Early trials |
| BRCA2 | splice_donor_variant | Complete Match | Rucaparib (PARP inhibitor) | Responsive | OV | FDA guidelines |
| BRCA2 | splice_donor_variant | Complete Match | PD1/PDL1 inhibitor | Responsive | UTH | Early trials |
| BRCA2 | splice_donor_variant | Complete Match | Veliparib;Cisplatin (PARP inhibitor;Chemotherapy) | Responsive | BRCA | Early trials |
| BRCA2 | splice_donor_variant | Complete Match | Platinum Agent (Chemotherapy) | Responsive | BRCA | Early trials |
| BRCA2 | splice_donor_variant | Complete Match | PARP inhibitor | Responsive | BRCA | Early trials |
| BRCA2 | splice_donor_variant | Different Alteration | PD1/PDL1 inhibitor | Responsive | UTH | Early trials |
| BRCA2 | splice_donor_variant | Different Alteration | PARP inhibitor | Responsive | OV | Pre-clinical |
| BRCA2 | W2970* | Complete Match | PD1 Ab inhibitor | Responsive | CM | Case report |
| BRCA2 | W2970* | Complete Match | Olaparib (PARP inhibitor) | Responsive | OV | FDA guidelines |
| BRCA2 | W2970* | Complete Match | Platinum Agent (Chemotherapy) | Responsive | OV | Late trials |
| BRCA2 | W2970* | Complete Match | Chemotherapy (PARP inhibitor;Chemotherapy) | Responsive | OV | Early trials |
| BRCA2 | W2970* | Complete Match | PARP inhibitor;Chemotherapy | Responsive | OV | Early trials |
| BRCA2 | W2970* | Complete Match | Platinum Agent (Chemotherapy) | Responsive | PA | Case report |
| BRCA2 | W2970* | Complete Match | Olaparib (PARP inhibitor) | Responsive | PRAD | Early trials |
| BRCA2 | W2970* | Complete Match | Rucaparib (PARP inhibitor) | Responsive | OV | FDA guidelines |
| BRCA2 | W2970* | Complete Match | PD1/PDL1 inhibitor | Responsive | UTH | Early trials |
| BRCA2 | W2970* | Complete Match | Veliparib;Cisplatin (PARP inhibitor;Chemotherapy) | Responsive | BRCA | Early trials |
| BRCA2 | W2970* | Complete Match | Platinum Agent (Chemotherapy) | Responsive | BRCA | Early trials |
| BRCA2 | W2970* | Complete Match | PARP inhibitor | Responsive | BRCA | Early trials |
| BRCA2 | W2970* | Different Alteration | PD1/PDL1 inhibitor | Responsive | UTH | Early trials |
| BRCA2 | W2970* | Different Alteration | PARP inhibitor | Responsive | OV | Pre-clinical |
| BRD4 | intron_variant | Different Alteration | BET inhibitor | Responsive | NMC | Case report |
| BRD4 | V583M | Different Alteration | BET inhibitor | Responsive | NMC | Case report |
| BRD4 | Q256P | Different Alteration | BET inhibitor | Responsive | NMC | Case report |
| CDKN2A | W15* | Complete Match | Ilorasertib (AURKA-VEGF inhibitor) | Responsive | CANCER | Early trials |
| CDKN2A | W15* | Complete Match | CDK4/6 inhibitor | Responsive | CANCER, G | Pre-clinical |
| CDKN2A | W15* | Complete Match | CDK4/6 inhibitor | Responsive | CM | Case report |
| CDKN2A | W15* | Complete Match | PD1 inhibitor | Resistant | CM | Early trials |
| CDKN2A | W15* | Different Alteration | CDK4/6 inhibitor | Responsive | CANCER, G | Pre-clinical |
| CDKN2A | W15* | Different Alteration | Ilorasertib (AURKA-VEGF inhibitor) | Responsive | CANCER | Early trials |
| CDKN2A | W15* | Different Alteration | PD1 inhibitor | Resistant | CM | Early trials |
| CDKN2A | W15* | Different Alteration | CDK4/6 inhibitor | Responsive | CM | Case report |
| CDKN2A | R80* | Complete Match | Ilorasertib (AURKA-VEGF inhibitor) | Responsive | CANCER | Early trials |
| CDKN2A | R80* | Complete Match | CDK4/6 inhibitor | Responsive | CANCER, G | Pre-clinical |
| CDKN2A | R80* | Complete Match | CDK4/6 inhibitor | Responsive | CM | Case report |
| CDKN2A | R80* | Complete Match | PD1 inhibitor | Resistant | CM | Early trials |
| CDKN2A | R80* | Different Alteration | CDK4/6 inhibitor | Responsive | CANCER, G | Pre-clinical |
| CDKN2A | R80* | Different Alteration | Ilorasertib (AURKA-VEGF inhibitor) | Responsive | CANCER | Early trials |
| CDKN2A | R80* | Different Alteration | PD1 inhibitor | Resistant | CM | Early trials |
| CDKN2A | R80* | Different Alteration | CDK4/6 inhibitor | Responsive | CM | Case report |
| CDKN2A | H83Y | Complete Match | Ilorasertib (AURKA-VEGF inhibitor) | Responsive | CANCER | Early trials |
| CDKN2A | H83Y | Complete Match | CDK4/6 inhibitor | Responsive | CANCER, G | Pre-clinical |
| CDKN2A | H83Y | Complete Match | CDK4/6 inhibitor | Responsive | CM | Case report |
| CDKN2A | H83Y | Complete Match | PD1 inhibitor | Resistant | CM | Early trials |
| CDKN2A | H83Y | Different Alteration | CDK4/6 inhibitor | Responsive | CANCER, G | Pre-clinical |
| CDKN2A | H83Y | Different Alteration | Ilorasertib (AURKA-VEGF inhibitor) | Responsive | CANCER | Early trials |
| CDKN2A | H83Y | Different Alteration | PD1 inhibitor | Resistant | CM | Early trials |
| CDKN2A | H83Y | Different Alteration | CDK4/6 inhibitor | Responsive | CM | Case report |
| CDKN2A | D108G | Complete Match | Ilorasertib (AURKA-VEGF inhibitor) | Responsive | CANCER | Early trials |
| CDKN2A | D108G | Complete Match | CDK4/6 inhibitor | Responsive | CANCER, G | Pre-clinical |
| CDKN2A | D108G | Complete Match | CDK4/6 inhibitor | Responsive | CM | Case report |
| CDKN2A | D108G | Complete Match | PD1 inhibitor | Resistant | CM | Early trials |
| CDKN2A | D108G | Different Alteration | CDK4/6 inhibitor | Responsive | CANCER, G | Pre-clinical |
| CDKN2A | D108G | Different Alteration | Ilorasertib (AURKA-VEGF inhibitor) | Responsive | CANCER | Early trials |
| CDKN2A | D108G | Different Alteration | PD1 inhibitor | Resistant | CM | Early trials |
| CDKN2A | D108G | Different Alteration | CDK4/6 inhibitor | Responsive | CM | Case report |
| CDKN2A | W110* | Complete Match | Ilorasertib (AURKA-VEGF inhibitor) | Responsive | CANCER | Early trials |
| CDKN2A | W110* | Complete Match | CDK4/6 inhibitor | Responsive | CANCER, G | Pre-clinical |
| CDKN2A | W110* | Complete Match | CDK4/6 inhibitor | Responsive | CM | Case report |
| CDKN2A | W110* | Complete Match | PD1 inhibitor | Resistant | CM | Early trials |
| CDKN2A | W110* | Different Alteration | CDK4/6 inhibitor | Responsive | CANCER, G | Pre-clinical |
| CDKN2A | W110* | Different Alteration | Ilorasertib (AURKA-VEGF inhibitor) | Responsive | CANCER | Early trials |
| CDKN2A | W110* | Different Alteration | PD1 inhibitor | Resistant | CM | Early trials |
| CDKN2A | W110* | Different Alteration | CDK4/6 inhibitor | Responsive | CM | Case report |
| CDKN2A | D108V | Complete Match | Ilorasertib (AURKA-VEGF inhibitor) | Responsive | CANCER | Early trials |
| CDKN2A | D108V | Complete Match | CDK4/6 inhibitor | Responsive | CANCER, G | Pre-clinical |
| CDKN2A | D108V | Complete Match | CDK4/6 inhibitor | Responsive | CM | Case report |
| CDKN2A | D108V | Complete Match | PD1 inhibitor | Resistant | CM | Early trials |
| CDKN2A | D108V | Different Alteration | CDK4/6 inhibitor | Responsive | CANCER, G | Pre-clinical |
| CDKN2A | D108V | Different Alteration | Ilorasertib (AURKA-VEGF inhibitor) | Responsive | CANCER | Early trials |
| CDKN2A | D108V | Different Alteration | PD1 inhibitor | Resistant | CM | Early trials |
| CDKN2A | D108V | Different Alteration | CDK4/6 inhibitor | Responsive | CM | Case report |
| CDKN2A | R58*), CDKN2A MUT (R80* | Complete Match | Ilorasertib (AURKA-VEGF inhibitor) | Responsive | CANCER | Early trials |
| CDKN2A | R58*), CDKN2A MUT (R80* | Complete Match | CDK4/6 inhibitor | Responsive | CANCER, G | Pre-clinical |
| CDKN2A | R58*), CDKN2A MUT (R80* | Complete Match | CDK4/6 inhibitor | Responsive | CM | Case report |
| CDKN2A | R58*), CDKN2A MUT (R80* | Complete Match | PD1 inhibitor | Resistant | CM | Early trials |
| CDKN2A | R58*), CDKN2A MUT (R80* | Different Alteration | CDK4/6 inhibitor | Responsive | CANCER, G | Pre-clinical |
| CDKN2A | R58*), CDKN2A MUT (R80* | Different Alteration | Ilorasertib (AURKA-VEGF inhibitor) | Responsive | CANCER | Early trials |
| CDKN2A | R58*), CDKN2A MUT (R80* | Different Alteration | PD1 inhibitor | Resistant | CM | Early trials |
| CDKN2A | R58*), CDKN2A MUT (R80* | Different Alteration | CDK4/6 inhibitor | Responsive | CM | Case report |
| CDKN2A | splice_acceptor_variant | Complete Match | Ilorasertib (AURKA-VEGF inhibitor) | Responsive | CANCER | Early trials |
| CDKN2A | splice_acceptor_variant | Complete Match | CDK4/6 inhibitor | Responsive | CANCER, G | Pre-clinical |
| CDKN2A | splice_acceptor_variant | Complete Match | CDK4/6 inhibitor | Responsive | CM | Case report |
| CDKN2A | splice_acceptor_variant | Complete Match | PD1 inhibitor | Resistant | CM | Early trials |
| CDKN2A | splice_acceptor_variant | Different Alteration | CDK4/6 inhibitor | Responsive | CANCER, G | Pre-clinical |
| CDKN2A | splice_acceptor_variant | Different Alteration | Ilorasertib (AURKA-VEGF inhibitor) | Responsive | CANCER | Early trials |
| CDKN2A | splice_acceptor_variant | Different Alteration | PD1 inhibitor | Resistant | CM | Early trials |
| CDKN2A | splice_acceptor_variant | Different Alteration | CDK4/6 inhibitor | Responsive | CM | Case report |
| CDKN2A | Y129* | Complete Match | Ilorasertib (AURKA-VEGF inhibitor) | Responsive | CANCER | Early trials |
| CDKN2A | Y129* | Complete Match | CDK4/6 inhibitor | Responsive | CANCER, G | Pre-clinical |
| CDKN2A | Y129* | Complete Match | CDK4/6 inhibitor | Responsive | CM | Case report |
| CDKN2A | Y129* | Complete Match | PD1 inhibitor | Resistant | CM | Early trials |
| CDKN2A | Y129* | Different Alteration | CDK4/6 inhibitor | Responsive | CANCER, G | Pre-clinical |
| CDKN2A | Y129* | Different Alteration | Ilorasertib (AURKA-VEGF inhibitor) | Responsive | CANCER | Early trials |
| CDKN2A | Y129* | Different Alteration | PD1 inhibitor | Resistant | CM | Early trials |
| CDKN2A | Y129* | Different Alteration | CDK4/6 inhibitor | Responsive | CM | Case report |
| CDKN2A | G111V | Complete Match | Ilorasertib (AURKA-VEGF inhibitor) | Responsive | CANCER | Early trials |
| CDKN2A | G111V | Complete Match | CDK4/6 inhibitor | Responsive | CANCER, G | Pre-clinical |
| CDKN2A | G111V | Complete Match | CDK4/6 inhibitor | Responsive | CM | Case report |
| CDKN2A | G111V | Complete Match | PD1 inhibitor | Resistant | CM | Early trials |
| CDKN2A | G111V | Different Alteration | CDK4/6 inhibitor | Responsive | CANCER, G | Pre-clinical |
| CDKN2A | G111V | Different Alteration | Ilorasertib (AURKA-VEGF inhibitor) | Responsive | CANCER | Early trials |
| CDKN2A | G111V | Different Alteration | PD1 inhibitor | Resistant | CM | Early trials |
| CDKN2A | G111V | Different Alteration | CDK4/6 inhibitor | Responsive | CM | Case report |
| CDKN2A | W110R | Complete Match | Ilorasertib (AURKA-VEGF inhibitor) | Responsive | CANCER | Early trials |
| CDKN2A | W110R | Complete Match | CDK4/6 inhibitor | Responsive | CANCER, G | Pre-clinical |
| CDKN2A | W110R | Complete Match | CDK4/6 inhibitor | Responsive | CM | Case report |
| CDKN2A | W110R | Complete Match | PD1 inhibitor | Resistant | CM | Early trials |
| CDKN2A | W110R | Different Alteration | CDK4/6 inhibitor | Responsive | CANCER, G | Pre-clinical |
| CDKN2A | W110R | Different Alteration | Ilorasertib (AURKA-VEGF inhibitor) | Responsive | CANCER | Early trials |
| CDKN2A | W110R | Different Alteration | PD1 inhibitor | Resistant | CM | Early trials |
| CDKN2A | W110R | Different Alteration | CDK4/6 inhibitor | Responsive | CM | Case report |
| CDKN2C | G36* | Complete Match | CDK2 inhibitor | Responsive | CANCER, G | Pre-clinical |
| CDKN2C | G36* | Different Alteration | CDK2 inhibitor | Responsive | G, CANCER | Pre-clinical |
| CRLF2 | intron_variant | Different Alteration | MTOR inhibitor | Responsive | ALL | Pre-clinical |
| CRLF2 | intron_variant | Different Alteration | BET inhibitor | Responsive | ALL | Pre-clinical |
| CRLF2 | intron_variant), CRLF2 MUT* (intron_variant | Different Alteration | MTOR inhibitor | Responsive | ALL | Pre-clinical |
| CRLF2 | intron_variant), CRLF2 MUT* (intron_variant | Different Alteration | BET inhibitor | Responsive | ALL | Pre-clinical |
| CTNNB1 | S37F | Different Mutation | Everolimus;Letrozole (MTOR inhibitor;Hormonal therapy) | Responsive | ED | Early trials |
| CTNNB1 | S37F | Complete Match | Tankyrase inhibitor | Resistant | COREAD | Pre-clinical |
| CTNNB1 | S45F | Different Mutation | Everolimus;Letrozole (MTOR inhibitor;Hormonal therapy) | Responsive | ED | Early trials |
| CTNNB1 | S45F | Complete Match | Tankyrase inhibitor | Resistant | COREAD | Pre-clinical |
| EGFR | intron_variant | Different Alteration | Erlotinib (EGFR inhibitor) | Responsive | L | Case report |
| EGFR | intron_variant | Different Alteration | Erlotinib (EGFR inhibitor) | Responsive | NSCLC | Case report |
| EGFR | IntronicBlockSubstitution | Different Alteration | Erlotinib (EGFR inhibitor) | Responsive | L | Case report |
| EGFR | IntronicBlockSubstitution | Different Alteration | Erlotinib (EGFR inhibitor) | Responsive | NSCLC | Case report |
| EGFR | R832C | Different Alteration | Cetuximab (EGFR mAb inhibitor) | Responsive | COREAD | FDA guidelines |
| EGFR | R832C | Different Alteration | Panitumumab (EGFR mAb inhibitor) | Resistant | COREAD | FDA guidelines |
| EGFR | R832C | Different Mutation | Rindopepimut (Vaccine) | Responsive | GB | Late trials |
| EGFR | R832C | Different Mutation | Afatinib (ERBB2 & EGFR inhibitor) | Responsive | NSCLC | FDA guidelines |
| EGFR | R832C | Different Mutation | Gefitinib (EGFR inhibitor) | Responsive | NSCLC | FDA guidelines |
| EGFR | R832C | Different Mutation | HSP90 inhibitor | Responsive | L | Early trials |
| EGFR | R832C | Different Mutation | Afatinib;Cetuximab (ERBB2 & EGFR inhibitor;EGFR mAb inhibitor) | Responsive | L | Early trials |
| EGFR | R832C | Different Mutation | MEK inhibitor | Responsive | L | Pre-clinical |
| EGFR | R832C | Different Mutation | EGFR TK inhibitor | Responsive | L | Late trials |
| EGFR | R832C | Different Mutation | Erlotinib (EGFR inhibitor) | Responsive | NSCLC | NCCN guidelines |
| EGFR | R832C | Different Mutation | Osimertinib (EGFR inhibitor) | Resistant | L | Early trials |
| EGFR | R832C | Different Mutation | EGFR-TKI's (EGFR inhibitor) | Resistant | L | Late trials |
| EGFR | R832C | Different Mutation | HSP90 inhibitor | Responsive | L | Case report |
| EGFR | R832C | Different Mutation | EGFR inhibitor | Resistant | L | Late trials |
| EGFR | R832C | Different Mutation | Poziotinib (EGFR inhibitor) | Responsive | L | Early trials |
| EGFR | R832C | Different Mutation | Osimertinib (EGFR inhibitor) | Responsive | L | Pre-clinical |
| EGFR | R832C | Different Mutation | HSP90 inhibitor (HSP90 inhibitor) | Responsive | L | Early trials |
| EGFR | R832C | Different Mutation | Erlotinib (EGFR inhibitor) | Responsive | G | Pre-clinical |
| EGFR | R832C | Different Mutation | Erlotinib (EGFR inhibitor) | Responsive | NSCLC | FDA guidelines |
| EGFR | R832C | Different Mutation | Osimertinib (EGFR inhibitor) | Responsive | L | Early trials |
| EGFR | R832C | Different Mutation | Rociletinib (EGFR inhibitor) | Resistant | LUAD | Case report |
| EGFR | R832C | Different Mutation | Panitumumab (EGFR mAb inhibitor) | Responsive | COREAD | Case report |
| EGFR | R832C | Different Mutation | novel EGFR mAb inhibitor | Responsive | COREAD | Early trials |
| EGFR | R832C | Different Mutation | Cetuximab (EGFR mAb inhibitor) | Resistant | COREAD | Case report |
| EGFR | R832C | Different Mutation | Cetuximab;Sirolimus (EGFR mAb inhibitor;MTOR inhibitor) | Responsive | HNC | Case report |
| EGFR | R832C | Different Mutation | Erlotinib (EGFR inhibitor) | Responsive | L | Case report |
| EGFR | R832C | Different Mutation | Gefitinib (EGFR inhibitor) | No Responsive | HNC | Case report |
| EGFR | R832C | Different Mutation | EGFR inhibitor | Responsive | L | Early trials |
| EGFR | R832C | Different Mutation | Erlotinib (EGFR inhibitor) | Resistant | L | NCCN/CAP guidelines |
| EGFR | R832C | Different Mutation | Osimertinib (EGFR inhibitor) | Responsive | NSCLC | FDA guidelines |
| EGFR | R832C | Different Mutation | EGFR inhibitor | Responsive | NSCLC | Late trials |
| EGFR | R832C | Different Mutation | Afatinib;Nimotuzumab (ERBB2 & EGFR inhibitor;EGFR mAb inhibitor) | Responsive | L | Early trials |
| EGFR | R832C | Different Mutation | Afatinib (ERBB2 & EGFR inhibitor) | Resistant | L | NCCN/CAP guidelines |
| EGFR | R832C | Different Mutation | Erlotinib (EGFR inhibitor) | Responsive | L | Early trials |
| EGFR | R832C | Different Mutation | Afatinib (ERBB2 & EGFR inhibitor) | Responsive | L | Late trials |
| EGFR | R832C | Different Mutation | Cetuximab (EGFR mAb inhibitor) | Resistant | COREAD | Pre-clinical |
| EGFR | R832C | Different Mutation | Erlotinib (EGFR inhibitor) | No Responsive | L | Case report |
| EGFR | R832C | Different Mutation | Lapatinib (ERBB2 inhibitor) | Responsive | ED | Case report |
| EGFR | R832C | Different Mutation | EGFR inhibitor | No Responsive | G | Early trials |
| EGFR | R832C | Different Mutation | EGFR inhibitor | Resistant | NSCLC | Case report |
| EGFR | R832C | Different Mutation | Panitumumab (EGFR mAb inhibitor) | Resistant | COREAD | Case report |
| EGFR | R832C | Different Mutation | Cetuximab (EGFR mAb inhibitor) | Resistant;Resistant | COREAD | Pre-clinical;Case report |
| EGFR | R832C | Different Mutation | Cetuximab (EGFR mAb inhibitor) | Responsive | HNC | Case report |
| EGFR | R832C | Different Alteration | Gefitinib (EGFR inhibitor) | Responsive | ED | Late trials |
| EGFR | R832C | Different Alteration | Gefitinib (EGFR inhibitor) | No Responsive | HNC | Early trials |
| EGFR | R832C | Different Alteration | EGFR inhibitor | Responsive | HNSC | Case report |
| EGFR | R832C | Different Alteration | EGFR inhibitor | No Responsive | G | Early trials |
| EGFR | R832C | Different Alteration | Erlotinib (EGFR inhibitor) | No Responsive | L | Early trials |
| EGFR | R832C | Different Alteration | EGFR mAb inhibitor | Responsive | COREAD | Late trials |
| EGFR | R832C | Different Alteration | Erlotinib (EGFR inhibitor) | Responsive | L | Case report |
| EGFR | R832C | Different Alteration | Erlotinib (EGFR inhibitor) | Responsive | NSCLC | Case report |
| EGFR | I569V | Different Alteration | Erlotinib (EGFR inhibitor) | Responsive | L | Case report |
| EGFR | I569V | Different Alteration | Erlotinib (EGFR inhibitor) | Responsive | NSCLC | Case report |
| EGFR | intron_variant), EGFR MUT* (intron_variant), EGFR MUT* (intron_variant | Different Alteration | Erlotinib (EGFR inhibitor) | Responsive | L | Case report |
| EGFR | intron_variant), EGFR MUT* (intron_variant), EGFR MUT* (intron_variant | Different Alteration | Erlotinib (EGFR inhibitor) | Responsive | NSCLC | Case report |
| EGFR | intron_variant), EGFR MUT* (intron_variant | Different Alteration | Erlotinib (EGFR inhibitor) | Responsive | L | Case report |
| EGFR | intron_variant), EGFR MUT* (intron_variant | Different Alteration | Erlotinib (EGFR inhibitor) | Responsive | NSCLC | Case report |
| EGFR | intron_variant), EGFR MUT* (C535C), EGFR MUT* (intron_variant), EGFR MUT* (intron_variant), EGFR MUT* (intron_variant), EGFR MUT* (intron_variant), EGFR MUT* (intron_variant | Different Alteration | Erlotinib (EGFR inhibitor) | Responsive | L | Case report |
| EGFR | intron_variant), EGFR MUT* (C535C), EGFR MUT* (intron_variant), EGFR MUT* (intron_variant), EGFR MUT* (intron_variant), EGFR MUT* (intron_variant), EGFR MUT* (intron_variant | Different Alteration | Erlotinib (EGFR inhibitor) | Responsive | NSCLC | Case report |
| EGFR | 3-UTRSNV | Different Alteration | Erlotinib (EGFR inhibitor) | Responsive | L | Case report |
| EGFR | 3-UTRSNV | Different Alteration | Erlotinib (EGFR inhibitor) | Responsive | NSCLC | Case report |
| EGFR | intron_variant), EGFR MUT* (intron_variant), EGFR MUT* (intron_variant), EGFR MUT* (intron_variant), EGFR MUT* (intron_variant), EGFR MUT* (intron_variant), EGFR MUT* (intron_variant), EGFR MUT* (intron_variant), EGFR MUT* (intron_variant), EGFR MUT* | Different Alteration | Erlotinib (EGFR inhibitor) | Responsive | L | Case report |
| EGFR | intron_variant), EGFR MUT* (intron_variant), EGFR MUT* (intron_variant), EGFR MUT* (intron_variant), EGFR MUT* (intron_variant), EGFR MUT* (intron_variant), EGFR MUT* (intron_variant), EGFR MUT* (intron_variant), EGFR MUT* (intron_variant), EGFR MUT* | Different Alteration | Erlotinib (EGFR inhibitor) | Responsive | NSCLC | Case report |
| EGFR | intron_variant), EGFR MUT* (intron_variant), EGFR MUT* (intron_variant), EGFR MUT* (intron_variant), EGFR MUT* (intron_variant), EGFR MUT* (intron_variant), EGFR MUT* (intron_variant | Different Alteration | Erlotinib (EGFR inhibitor) | Responsive | L | Case report |
| EGFR | intron_variant), EGFR MUT* (intron_variant), EGFR MUT* (intron_variant), EGFR MUT* (intron_variant), EGFR MUT* (intron_variant), EGFR MUT* (intron_variant), EGFR MUT* (intron_variant | Different Alteration | Erlotinib (EGFR inhibitor) | Responsive | NSCLC | Case report |
| EGFR | A1201A), EGFR MUT* (intron_variant), EGFR MUT* (intron_variant), EGFR MUT* (intron_variant | Different Alteration | Erlotinib (EGFR inhibitor) | Responsive | L | Case report |
| EGFR | A1201A), EGFR MUT* (intron_variant), EGFR MUT* (intron_variant), EGFR MUT* (intron_variant | Different Alteration | Erlotinib (EGFR inhibitor) | Responsive | NSCLC | Case report |
| EGFR | intron_variant), EGFR MUT* (intron_variant), EGFR MUT* (intron_variant), EGFR MUT* (intron_variant), EGFR MUT* (intron_variant | Different Alteration | Erlotinib (EGFR inhibitor) | Responsive | L | Case report |
| EGFR | intron_variant), EGFR MUT* (intron_variant), EGFR MUT* (intron_variant), EGFR MUT* (intron_variant), EGFR MUT* (intron_variant | Different Alteration | Erlotinib (EGFR inhibitor) | Responsive | NSCLC | Case report |
| EPHA3 | R745* | Different Alteration | EPHA3 inhibitor | Responsive | CANCER | Pre-clinical |
| ERBB4 | intron_variant), ERBB4 MUT* (intron_variant), ERBB4 MUT* (intron_variant), ERBB4 MUT* (intron_variant), ERBB4 MUT* (intron_variant), ERBB4 MUT* (intron_variant), ERBB4 MUT* (intron_variant), ERBB4 MUT* (intron_variant | Different Alteration | Lapatinib;Afatinib (ERBB2 inhibitor;ERBB2 & EGFR inhibitor) | Responsive | LUAD | Pre-clinical |
| ERBB4 | intron_variant), ERBB4 MUT* (intron_variant), ERBB4 MUT* (intron_variant), ERBB4 MUT* (intron_variant), ERBB4 MUT* (intron_variant), ERBB4 MUT* (intron_variant | Different Alteration | Lapatinib;Afatinib (ERBB2 inhibitor;ERBB2 & EGFR inhibitor) | Responsive | LUAD | Pre-clinical |
| ERBB4 | intron_variant), ERBB4 MUT* (intron_variant | Different Alteration | Lapatinib;Afatinib (ERBB2 inhibitor;ERBB2 & EGFR inhibitor) | Responsive | LUAD | Pre-clinical |
| ERBB4 | intron_variant), ERBB4 MUT* (intron_variant), ERBB4 MUT* (intron_variant), ERBB4 MUT* (intron_variant), ERBB4 MUT* (intron_variant), ERBB4 MUT* (intron_variant), ERBB4 MUT* (intron_variant), ERBB4 MUT* (intron_variant), ERBB4 MUT* (intron_variant), E | Different Alteration | Lapatinib;Afatinib (ERBB2 inhibitor;ERBB2 & EGFR inhibitor) | Responsive | LUAD | Pre-clinical |
| ERBB4 | intron_variant), ERBB4 MUT* (intron_variant), ERBB4 MUT* (intron_variant), ERBB4 MUT* (intron_variant | Different Alteration | Lapatinib;Afatinib (ERBB2 inhibitor;ERBB2 & EGFR inhibitor) | Responsive | LUAD | Pre-clinical |
| ERBB4 | intron_variant), ERBB4 MUT* (intron_variant), ERBB4 MUT* (intron_variant), ERBB4 MUT* (intron_variant), ERBB4 MUT* (intron_variant), ERBB4 MUT* (intron_variant), ERBB4 MUT* (intron_variant), ERBB4 MUT* (intron_variant), ERBB4 MUT* (intron_variant | Different Alteration | Lapatinib;Afatinib (ERBB2 inhibitor;ERBB2 & EGFR inhibitor) | Responsive | LUAD | Pre-clinical |
| ERBB4 | intron_variant), ERBB4 MUT* (intron_variant), ERBB4 MUT* (intron_variant | Different Alteration | Lapatinib;Afatinib (ERBB2 inhibitor;ERBB2 & EGFR inhibitor) | Responsive | LUAD | Pre-clinical |
| ERBB4 | intron_variant), ERBB4 MUT* (intron_variant), ERBB4 MUT* (intron_variant), ERBB4 MUT* (intron_variant), ERBB4 MUT* (intron_variant), ERBB4 MUT* (intron_variant), ERBB4 MUT* (intron_variant | Different Alteration | Lapatinib;Afatinib (ERBB2 inhibitor;ERBB2 & EGFR inhibitor) | Responsive | LUAD | Pre-clinical |
| ERBB4 | intron_variant | Different Alteration | Lapatinib;Afatinib (ERBB2 inhibitor;ERBB2 & EGFR inhibitor) | Responsive | LUAD | Pre-clinical |
| ERBB4 | intron_variant), ERBB4 MUT* (intron_variant), ERBB4 MUT* (intron_variant), ERBB4 MUT* (intron_variant), ERBB4 MUT* (intron_variant | Different Alteration | Lapatinib;Afatinib (ERBB2 inhibitor;ERBB2 & EGFR inhibitor) | Responsive | LUAD | Pre-clinical |
| ERBB4 | intron_variant), ERBB4 MUT* (intron_variant), ERBB4 MUT* (intron_variant), ERBB4 MUT* (intron_variant), ERBB4 MUT* (intron_variant), ERBB4 MUT* (3-UTRSNV | Different Alteration | Lapatinib;Afatinib (ERBB2 inhibitor;ERBB2 & EGFR inhibitor) | Responsive | LUAD | Pre-clinical |
| ERBB4 | intron_variant), ERBB4 MUT* (intron_variant), ERBB4 MUT* (intron_variant), ERBB4 MUT* (intron_variant), ERBB4 MUT* (intron_variant), ERBB4 MUT* (intron_variant), ERBB4 MUT* (T731T | Different Alteration | Lapatinib;Afatinib (ERBB2 inhibitor;ERBB2 & EGFR inhibitor) | Responsive | LUAD | Pre-clinical |
| ERBB4 | R992C | Different Alteration | Lapatinib;Afatinib (ERBB2 inhibitor;ERBB2 & EGFR inhibitor) | Responsive | LUAD | Pre-clinical |
| ERBB4 | intron_variant), ERBB4 MUT* (intron_variant), ERBB4 MUT* (intron_variant), ERBB4 MUT* (intron_variant), ERBB4 MUT* (intron_variant), ERBB4 MUT* (intron_variant), ERBB4 MUT* (3-UTRSNV | Different Alteration | Lapatinib;Afatinib (ERBB2 inhibitor;ERBB2 & EGFR inhibitor) | Responsive | LUAD | Pre-clinical |
| ERBB4 | intron_variant), ERBB4 MUT* (intron_variant), ERBB4 MUT* (G702G | Different Alteration | Lapatinib;Afatinib (ERBB2 inhibitor;ERBB2 & EGFR inhibitor) | Responsive | LUAD | Pre-clinical |
| ERBB4 | intron_variant), ERBB4 MUT* (M250I | Different Alteration | Lapatinib;Afatinib (ERBB2 inhibitor;ERBB2 & EGFR inhibitor) | Responsive | LUAD | Pre-clinical |
| ERBB4 | intron_variant), ERBB4 MUT* (intron_variant), ERBB4 MUT* (intron_variant), ERBB4 MUT* (R782Q | Different Alteration | Lapatinib;Afatinib (ERBB2 inhibitor;ERBB2 & EGFR inhibitor) | Responsive | LUAD | Pre-clinical |
| ERBB4 | Q707* | Different Mutation | Lapatinib (ERBB2 inhibitor) | Responsive | CM | Pre-clinical |
| ERBB4 | Q707* | Different Mutation | ERBB2 inhibitor | Resistant | BRCA | Case report |
| ERBB4 | Q707*), ERBB4 MUT* (intron_variant), ERBB4 MUT* (intron_variant | Different Alteration | Lapatinib;Afatinib (ERBB2 inhibitor;ERBB2 & EGFR inhibitor) | Responsive | LUAD | Pre-clinical |
| ERBB4 | intron_variant), ERBB4 MUT* (intron_variant), ERBB4 MUT* (intron_variant), ERBB4 MUT* (intron_variant), ERBB4 MUT* (3-UTRSNV | Different Alteration | Lapatinib;Afatinib (ERBB2 inhibitor;ERBB2 & EGFR inhibitor) | Responsive | LUAD | Pre-clinical |
| ERCC2 | splice_donor_variant | Complete Match | Cisplatin (Chemotherapy) | Responsive | BLCA | Early trials |
| ERCC2 | splice_donor_variant | Complete Match | PD1/PDL1 inhibitor | Responsive | UTH | Early trials |
| ERCC2 | splice_donor_variant | Different Alteration | PD1/PDL1 inhibitor | Responsive | UTH | Early trials |
| ESR1 | intron_variant), ESR1 MUT* (intron_variant | Different Alteration | ESR1 inhibitor | Resistant | BRCA | Pre-clinical |
| ESR1 | intron_variant), ESR1 MUT* (intron_variant), ESR1 MUT* (intron_variant | Different Alteration | ESR1 inhibitor | Resistant | BRCA | Pre-clinical |
| ESR1 | intron_variant), ESR1 MUT* (IntronicBlockSubstitution | Different Alteration | ESR1 inhibitor | Resistant | BRCA | Pre-clinical |
| ESR1 | intron_variant | Different Alteration | ESR1 inhibitor | Resistant | BRCA | Pre-clinical |
| ESR1 | intron_variant), ESR1 MUT* (intron_variant), ESR1 MUT* (intron_variant), ESR1 MUT* (intron_variant), ESR1 MUT* (intron_variant | Different Alteration | ESR1 inhibitor | Resistant | BRCA | Pre-clinical |
| ESR1 | intron_variant), ESR1 MUT* (intron_variant), ESR1 MUT* (intron_variant), ESR1 MUT* (intron_variant | Different Alteration | ESR1 inhibitor | Resistant | BRCA | Pre-clinical |
| ESR1 | IntronicBlockSubstitution), ESR1 MUT* (intron_variant), ESR1 MUT* (intron_variant | Different Alteration | ESR1 inhibitor | Resistant | BRCA | Pre-clinical |
| ESR1 | intron_variant), ESR1 MUT* (intron_variant), ESR1 MUT* (intron_variant), ESR1 MUT* (intron_variant), ESR1 MUT* (intron_variant), ESR1 MUT* (intron_variant), ESR1 MUT* (intron_variant), ESR1 MUT* (intron_variant), ESR1 MUT* (intron_variant), ESR1 MUT* | Different Alteration | ESR1 inhibitor | Resistant | BRCA | Pre-clinical |
| ESR1 | E444E | Different Alteration | ESR1 inhibitor | Resistant | BRCA | Pre-clinical |
| ESR1 | intron_variant), ESR1 MUT* (intron_variant), ESR1 MUT* (intron_variant), ESR1 MUT* (intron_variant), ESR1 MUT* (intron_variant), ESR1 MUT* (intron_variant), ESR1 MUT* (intron_variant), ESR1 MUT* (IntronicBlockSubstitution), ESR1 MUT* (intron_variant) | Different Alteration | ESR1 inhibitor | Resistant | BRCA | Pre-clinical |
| ESR1 | T563M), ESR1 MUT* (intron_variant), ESR1 MUT* (intron_variant | Different Alteration | ESR1 inhibitor | Resistant | BRCA | Pre-clinical |
| ESR1 | intron_variant), ESR1 MUT* (IntronicBlockSubstitution), ESR1 MUT* (intron_variant), ESR1 MUT* (IntronicBlockSubstitution), ESR1 MUT* (intron_variant), ESR1 MUT* (intron_variant), ESR1 MUT* (intron_variant | Different Alteration | ESR1 inhibitor | Resistant | BRCA | Pre-clinical |
| ESR1 | IntronicBlockSubstitution), ESR1 MUT* (intron_variant), ESR1 MUT* (intron_variant), ESR1 MUT* (intron_variant | Different Alteration | ESR1 inhibitor | Resistant | BRCA | Pre-clinical |
| ESR1 | M388I), ESR1 MUT* (intron_variant | Different Alteration | ESR1 inhibitor | Resistant | BRCA | Pre-clinical |
| ESR1 | IntronicBlockSubstitution), ESR1 MUT* (IntronicBlockSubstitution), ESR1 MUT* (IntronicBlockSubstitution), ESR1 MUT* (IntronicBlockSubstitution | Different Alteration | ESR1 inhibitor | Resistant | BRCA | Pre-clinical |
| ESR1 | 3-UTRSNV), ESR1 MUT* (P336A | Different Alteration | ESR1 inhibitor | Resistant | BRCA | Pre-clinical |
| FBXW7 | R505C | Complete Match | Steroid | Responsive | ALL | Late trials |
| FBXW7 | R505C | Complete Match | Tubulin inhibitor | Resistant | CANCER | Pre-clinical |
| FBXW7 | R505C | Different Alteration | Sirolimus (MTOR inhibitor) | Responsive | COREAD | Pre-clinical |
| FBXW7 | R505C | Different Alteration | Tubulin inhibitor | Resistant | CANCER | Pre-clinical |
| FBXW7 | splice_acceptor_variant | Complete Match | Steroid | Responsive | ALL | Late trials |
| FBXW7 | splice_acceptor_variant | Complete Match | Tubulin inhibitor | Resistant | CANCER | Pre-clinical |
| FBXW7 | splice_acceptor_variant | Different Alteration | Sirolimus (MTOR inhibitor) | Responsive | COREAD | Pre-clinical |
| FBXW7 | splice_acceptor_variant | Different Alteration | Tubulin inhibitor | Resistant | CANCER | Pre-clinical |
| FBXW7 | Q42* | Complete Match | Steroid | Responsive | ALL | Late trials |
| FBXW7 | Q42* | Complete Match | Tubulin inhibitor | Resistant | CANCER | Pre-clinical |
| FBXW7 | Q42* | Different Alteration | Sirolimus (MTOR inhibitor) | Responsive | COREAD | Pre-clinical |
| FBXW7 | Q42* | Different Alteration | Tubulin inhibitor | Resistant | CANCER | Pre-clinical |
| FGFR2 | intron_variant | Different Alteration | FGFR inhibitor | Responsive | COREAD | Early trials |
| FGFR2 | intron_variant | Different Alteration | FGFR inhibitor | Responsive | BT | Early trials |
| FGFR2 | intron_variant), FGFR2 MUT* (intron_variant), FGFR2 MUT* (intron_variant | Different Alteration | FGFR inhibitor | Responsive | COREAD | Early trials |
| FGFR2 | intron_variant), FGFR2 MUT* (intron_variant), FGFR2 MUT* (intron_variant | Different Alteration | FGFR inhibitor | Responsive | BT | Early trials |
| FGFR2 | intron_variant), FGFR2 MUT* (intron_variant | Different Alteration | FGFR inhibitor | Responsive | COREAD | Early trials |
| FGFR2 | intron_variant), FGFR2 MUT* (intron_variant | Different Alteration | FGFR inhibitor | Responsive | BT | Early trials |
| FGFR2 | G80G | Different Alteration | FGFR inhibitor | Responsive | COREAD | Early trials |
| FGFR2 | G80G | Different Alteration | FGFR inhibitor | Responsive | BT | Early trials |
| FGFR3 | intron_variant | Different Alteration | FGFR inhibitor | Responsive | BLCA | Case report |
| FGFR3 | intron_variant | Different Alteration | FGFR inhibitor | Responsive | G | Early trials |
| FGFR3 | intron_variant | Different Alteration | FGFR inhibitor | Responsive | NSCLC | Pre-clinical |
| FGFR3 | A168A | Different Alteration | FGFR inhibitor | Responsive | BLCA | Case report |
| FGFR3 | A168A | Different Alteration | FGFR inhibitor | Responsive | G | Early trials |
| FGFR3 | A168A | Different Alteration | FGFR inhibitor | Responsive | NSCLC | Pre-clinical |
| FGFR3 | P39L | Different Alteration | FGFR inhibitor | Responsive | BLCA | Case report |
| FGFR3 | P39L | Different Alteration | FGFR inhibitor | Responsive | G | Early trials |
| FGFR3 | P39L | Different Alteration | FGFR inhibitor | Responsive | NSCLC | Pre-clinical |
| FGFR3 | 5-UTRSNV | Different Alteration | FGFR inhibitor | Responsive | BLCA | Case report |
| FGFR3 | 5-UTRSNV | Different Alteration | FGFR inhibitor | Responsive | G | Early trials |
| FGFR3 | 5-UTRSNV | Different Alteration | FGFR inhibitor | Responsive | NSCLC | Pre-clinical |
| GNAS | R201H | Complete Match | JAK inhibitor | Responsive | CANCER | Pre-clinical |
| GNAS | R201C | Complete Match | JAK inhibitor | Responsive | CANCER | Pre-clinical |
| JAK2 | intron_variant | Different Alteration | Ruxolitinib (JAK inhibitor) | Responsive | ALL | Pre-clinical |
| JAK2 | intron_variant), JAK2 MUT* (intron_variant | Different Alteration | Ruxolitinib (JAK inhibitor) | Responsive | ALL | Pre-clinical |
| JAK2 | IntronicBlockSubstitution | Different Alteration | Ruxolitinib (JAK inhibitor) | Responsive | ALL | Pre-clinical |
| JAK2 | IntronicBlockSubstitution), JAK2 MUT* (IntronicBlockSubstitution | Different Alteration | Ruxolitinib (JAK inhibitor) | Responsive | ALL | Pre-clinical |
| JAK2 | 3-UTRBlockSubstitution), JAK2 MUT* (intron_variant), JAK2 MUT* (intron_variant), JAK2 MUT* (intron_variant), JAK2 MUT* (intron_variant), JAK2 MUT* (intron_variant), JAK2 MUT* (intron_variant | Different Alteration | Ruxolitinib (JAK inhibitor) | Responsive | ALL | Pre-clinical |
| JAK2 | intron_variant), JAK2 MUT* (intron_variant), JAK2 MUT* (intron_variant | Different Alteration | Ruxolitinib (JAK inhibitor) | Responsive | ALL | Pre-clinical |
| JAK2 | intron_variant), JAK2 MUT* (intron_variant), JAK2 MUT* (intron_variant), JAK2 MUT* (intron_variant | Different Alteration | Ruxolitinib (JAK inhibitor) | Responsive | ALL | Pre-clinical |
| KMT2A | E2419E | Different Alteration | EPZ-5676 (DOT1L inhibitor) | Responsive | ALL, MDS, AML | Early trials |
| KMT2A | E2419E | Different Alteration | BET inhibitor | Responsive | ALL | Pre-clinical |
| KMT2A | E2419E | Different Alteration | BET inhibitor | Responsive | AML | Pre-clinical |
| KMT2A | E2419E | Different Alteration | Daunorubicin (Chemotherapy) | Responsive | AML | FDA guidelines |
| KMT2A | E2419E | Different Alteration | Belinostat (HDAC inhibitor) | Responsive | AML | Early trials |
| KMT2A | E2419E | Different Alteration | DOT1L inhibitor | Responsive | AML | Early trials |
| KMT2A | intron_variant | Different Alteration | EPZ-5676 (DOT1L inhibitor) | Responsive | ALL, MDS, AML | Early trials |
| KMT2A | intron_variant | Different Alteration | BET inhibitor | Responsive | ALL | Pre-clinical |
| KMT2A | intron_variant | Different Alteration | BET inhibitor | Responsive | AML | Pre-clinical |
| KMT2A | intron_variant | Different Alteration | Daunorubicin (Chemotherapy) | Responsive | AML | FDA guidelines |
| KMT2A | intron_variant | Different Alteration | Belinostat (HDAC inhibitor) | Responsive | AML | Early trials |
| KMT2A | intron_variant | Different Alteration | DOT1L inhibitor | Responsive | AML | Early trials |
| KMT2A | Q2206L | Different Alteration | EPZ-5676 (DOT1L inhibitor) | Responsive | ALL, MDS, AML | Early trials |
| KMT2A | Q2206L | Different Alteration | BET inhibitor | Responsive | ALL | Pre-clinical |
| KMT2A | Q2206L | Different Alteration | BET inhibitor | Responsive | AML | Pre-clinical |
| KMT2A | Q2206L | Different Alteration | Daunorubicin (Chemotherapy) | Responsive | AML | FDA guidelines |
| KMT2A | Q2206L | Different Alteration | Belinostat (HDAC inhibitor) | Responsive | AML | Early trials |
| KMT2A | Q2206L | Different Alteration | DOT1L inhibitor | Responsive | AML | Early trials |
| KMT2A | intron_variant), MLL MUT* (intron_variant | Different Alteration | EPZ-5676 (DOT1L inhibitor) | Responsive | ALL, MDS, AML | Early trials |
| KMT2A | intron_variant), MLL MUT* (intron_variant | Different Alteration | BET inhibitor | Responsive | ALL | Pre-clinical |
| KMT2A | intron_variant), MLL MUT* (intron_variant | Different Alteration | BET inhibitor | Responsive | AML | Pre-clinical |
| KMT2A | intron_variant), MLL MUT* (intron_variant | Different Alteration | Daunorubicin (Chemotherapy) | Responsive | AML | FDA guidelines |
| KMT2A | intron_variant), MLL MUT* (intron_variant | Different Alteration | Belinostat (HDAC inhibitor) | Responsive | AML | Early trials |
| KMT2A | intron_variant), MLL MUT* (intron_variant | Different Alteration | DOT1L inhibitor | Responsive | AML | Early trials |
| KMT2A | intron_variant), MLL MUT* (intron_variant), MLL MUT* (intron_variant | Different Alteration | EPZ-5676 (DOT1L inhibitor) | Responsive | ALL, MDS, AML | Early trials |
| KMT2A | intron_variant), MLL MUT* (intron_variant), MLL MUT* (intron_variant | Different Alteration | BET inhibitor | Responsive | ALL | Pre-clinical |
| KMT2A | intron_variant), MLL MUT* (intron_variant), MLL MUT* (intron_variant | Different Alteration | BET inhibitor | Responsive | AML | Pre-clinical |
| KMT2A | intron_variant), MLL MUT* (intron_variant), MLL MUT* (intron_variant | Different Alteration | Daunorubicin (Chemotherapy) | Responsive | AML | FDA guidelines |
| KMT2A | intron_variant), MLL MUT* (intron_variant), MLL MUT* (intron_variant | Different Alteration | Belinostat (HDAC inhibitor) | Responsive | AML | Early trials |
| KMT2A | intron_variant), MLL MUT* (intron_variant), MLL MUT* (intron_variant | Different Alteration | DOT1L inhibitor | Responsive | AML | Early trials |
| KMT2A | K2434E | Different Alteration | EPZ-5676 (DOT1L inhibitor) | Responsive | ALL, MDS, AML | Early trials |
| KMT2A | K2434E | Different Alteration | BET inhibitor | Responsive | ALL | Pre-clinical |
| KMT2A | K2434E | Different Alteration | BET inhibitor | Responsive | AML | Pre-clinical |
| KMT2A | K2434E | Different Alteration | Daunorubicin (Chemotherapy) | Responsive | AML | FDA guidelines |
| KMT2A | K2434E | Different Alteration | Belinostat (HDAC inhibitor) | Responsive | AML | Early trials |
| KMT2A | K2434E | Different Alteration | DOT1L inhibitor | Responsive | AML | Early trials |
| KMT2A | P3515L | Different Alteration | EPZ-5676 (DOT1L inhibitor) | Responsive | ALL, MDS, AML | Early trials |
| KMT2A | P3515L | Different Alteration | BET inhibitor | Responsive | ALL | Pre-clinical |
| KMT2A | P3515L | Different Alteration | BET inhibitor | Responsive | AML | Pre-clinical |
| KMT2A | P3515L | Different Alteration | Daunorubicin (Chemotherapy) | Responsive | AML | FDA guidelines |
| KMT2A | P3515L | Different Alteration | Belinostat (HDAC inhibitor) | Responsive | AML | Early trials |
| KMT2A | P3515L | Different Alteration | DOT1L inhibitor | Responsive | AML | Early trials |
| KMT2A | 3-UTRSNV | Different Alteration | EPZ-5676 (DOT1L inhibitor) | Responsive | ALL, MDS, AML | Early trials |
| KMT2A | 3-UTRSNV | Different Alteration | BET inhibitor | Responsive | ALL | Pre-clinical |
| KMT2A | 3-UTRSNV | Different Alteration | BET inhibitor | Responsive | AML | Pre-clinical |
| KMT2A | 3-UTRSNV | Different Alteration | Daunorubicin (Chemotherapy) | Responsive | AML | FDA guidelines |
| KMT2A | 3-UTRSNV | Different Alteration | Belinostat (HDAC inhibitor) | Responsive | AML | Early trials |
| KMT2A | 3-UTRSNV | Different Alteration | DOT1L inhibitor | Responsive | AML | Early trials |
| KMT2A | IntronicBlockSubstitution | Different Alteration | EPZ-5676 (DOT1L inhibitor) | Responsive | ALL, MDS, AML | Early trials |
| KMT2A | IntronicBlockSubstitution | Different Alteration | BET inhibitor | Responsive | ALL | Pre-clinical |
| KMT2A | IntronicBlockSubstitution | Different Alteration | BET inhibitor | Responsive | AML | Pre-clinical |
| KMT2A | IntronicBlockSubstitution | Different Alteration | Daunorubicin (Chemotherapy) | Responsive | AML | FDA guidelines |
| KMT2A | IntronicBlockSubstitution | Different Alteration | Belinostat (HDAC inhibitor) | Responsive | AML | Early trials |
| KMT2A | IntronicBlockSubstitution | Different Alteration | DOT1L inhibitor | Responsive | AML | Early trials |
| KMT2A | L281L | Different Alteration | EPZ-5676 (DOT1L inhibitor) | Responsive | ALL, MDS, AML | Early trials |
| KMT2A | L281L | Different Alteration | BET inhibitor | Responsive | ALL | Pre-clinical |
| KMT2A | L281L | Different Alteration | BET inhibitor | Responsive | AML | Pre-clinical |
| KMT2A | L281L | Different Alteration | Daunorubicin (Chemotherapy) | Responsive | AML | FDA guidelines |
| KMT2A | L281L | Different Alteration | Belinostat (HDAC inhibitor) | Responsive | AML | Early trials |
| KMT2A | L281L | Different Alteration | DOT1L inhibitor | Responsive | AML | Early trials |
| KMT2D | R5282*), MLL2 MUT (splice_acceptor_variant | Complete Match | Bicalutamide (AR inhibitor) | Responsive | LUSC | Pre-clinical |
| KMT2D | splice_acceptor_variant | Complete Match | Bicalutamide (AR inhibitor) | Responsive | LUSC | Pre-clinical |
| KMT2D | E3068* | Complete Match | Bicalutamide (AR inhibitor) | Responsive | LUSC | Pre-clinical |
| KMT2D | E5507* | Complete Match | Bicalutamide (AR inhibitor) | Responsive | LUSC | Pre-clinical |
| KRAS | G12R | Complete Match | Panitumumab (EGFR mAb inhibitor) | Resistant | COREAD | FDA guidelines |
| KRAS | G12R | Complete Match | Cetuximab (EGFR mAb inhibitor) | Resistant | COREAD | FDA guidelines |
| KRAS | G12R | Different Mutation | Cetuximab (EGFR mAb inhibitor) | No Responsive | COREAD | Late trials |
| KRAS | G12R | Complete Match | MEK inhibitor | Responsive | ALL | Pre-clinical |
| KRAS | G12R | Complete Match | Imatinib (BCR-ABL inhibitor & KIT inhibitor) | Resistant | GIST | Case report |
| KRAS | G12R | Complete Match | MEK inhibitor | Responsive | OV, CER, AML | Pre-clinical |
| KRAS | G12R | Complete Match | Abemaciclib (CDK4/6 inhibitor) | Responsive | L | Early trials |
| KRAS | G12R | Complete Match | MEK inhibitor;PI3K pathway inhibitor | Responsive | COREAD | Pre-clinical |
| KRAS | G12R | Complete Match | Cetuximab (EGFR mAb inhibitor) | Resistant | COREAD | NCCN guidelines |
| KRAS | G12R | Complete Match | Sorafenib;MEK inhibitor (Pan-TK inhibitor;MEK inhibitor) | Responsive | HC | Early trials |
| KRAS | G12R | Complete Match | EGFR inhibitor | Resistant | L | NCCN guidelines |
| KRAS | G12R | Complete Match | CDK4 inhibitor | Responsive | L | Pre-clinical |
| KRAS | G12R | Complete Match | Selumetinib (MEK inhibitor) | Responsive | NSCLC | Early trials |
| KRAS | G12R | Complete Match | BET inhibitor | Responsive | L | Pre-clinical |
| KRAS | G12R | Complete Match | Decitabine;BCL2 inhibitor (Chemotherapy;BCL2 inhibitor) | Responsive | OV | Pre-clinical |
| KRAS | G12R | Complete Match | FAK inhibitor | Responsive | L | Pre-clinical |
| KRAS | G12R | Complete Match | EGFR inhibitor | Resistant | NSCLC | Pre-clinical |
| KRAS | G12R | Complete Match | EZH2 inhibitor | Resistant | CANCER | Pre-clinical |
| KRAS | G12R | Complete Match | MEK inhibitor | Responsive | BT, L | Early trials |
| KRAS | G12R | Complete Match | MEK inhibitor;IGF1R inhibitor | Responsive | COREAD | Pre-clinical |
| KRAS | G12R | Complete Match | MTOR inhibitor;BH3 mimetics | Responsive | COREAD | Pre-clinical |
| KRAS | G12R | Complete Match | EGFR mAb inhibitor;MEK inhibitor | Responsive | COREAD | Pre-clinical |
| KRAS | G12R | Complete Match | pan-RAF inhibitor | Responsive | ED | Case report |
| KRAS | G12R | Complete Match | EGFR mAb inhibitor | Resistant | ST | Pre-clinical |
| KRAS | G12R | Complete Match | PI3K pathway inhibitor;MEK inhibitor | Responsive | L | Early trials |
| KRAS | G12R | Complete Match | Selumetinib (MEK inhibitor) | No Responsive | L | Early trials |
| KRAS | G12R | Complete Match | CDK4/6 inhibitor;MEK inhibitor | Responsive | L | Early trials |
| KRAS | G12R | Complete Match | Trametinib;Ponatinib (MEK inhibitor;BCR-ABL inhibitor & Pan-TK inhibitor) | Responsive | LUAD | Pre-clinical |
| KRAS | G12R | Complete Match | Trastuzumab;Lapatinib (ERBB2 mAb inhibitor;ERBB2 inhibitor) | Resistant | COREAD | Late trials |
| KRAS | G12R | Complete Match | pan-RAF inhibitor | Responsive | L | Early trials |
| KRAS | G12R | Complete Match | JAK/TBK1/IKKε inhibitor | Responsive | L | Pre-clinical |
| KRAS | G12R | Complete Match | FAS inhibitor | Responsive | L | Case report |
| KRAS | G12R | Complete Match | HSP90 inhibitor | Responsive | L | Pre-clinical |
| KRAS | G12R | Complete Match | ERK inhibitor | Responsive | COREAD | Pre-clinical |
| KRAS | G12R | Complete Match | PI3K pathway inhibitor;MEK inhibitor | No Responsive | PA | Early trials |
| KRAS | G12R | Complete Match | MEK inhibitor;BCL-XL inhibitor | Responsive | COREAD | Pre-clinical |
| KRAS | G12R | Complete Match | Gemcitabine;MEK inhibitor (Chemotherapy;MEK inhibitor) | Responsive | PA | Early trials |
| KRAS | G12R | Complete Match | Panitumumab (EGFR mAb inhibitor) | Resistant | COREAD | NCCN guidelines |
| KRAS | G12R | Complete Match | PI3K pathway inhibitor | Resistant | ED | Pre-clinical |
| KRAS | G12R | Complete Match | CDK4/6 inhibitor;MEK inhibitor | Responsive | CESC | Pre-clinical |
| KRAS | G12R | Different Alteration | BRAF inhibitor;MEK inhibitor | Resistant | COREAD | Case report |
| KRAS | G12R | Different Alteration | BRAF inhibitor;EGFR mAb inhibitor | Resistant | COREAD | Case report |
| KRAS | G12D | Complete Match | Panitumumab (EGFR mAb inhibitor) | Resistant | COREAD | FDA guidelines |
| KRAS | G12D | Complete Match | Cetuximab (EGFR mAb inhibitor) | Resistant | COREAD | FDA guidelines |
| KRAS | G12D | Different Mutation | Cetuximab (EGFR mAb inhibitor) | No Responsive | COREAD | Late trials |
| KRAS | G12D | Complete Match | MEK inhibitor | Responsive | ALL | Pre-clinical |
| KRAS | G12D | Complete Match | Imatinib (BCR-ABL inhibitor & KIT inhibitor) | Resistant | GIST | Case report |
| KRAS | G12D | Complete Match | MEK inhibitor | Responsive | OV, CER, AML | Pre-clinical |
| KRAS | G12D | Complete Match | Abemaciclib (CDK4/6 inhibitor) | Responsive | L | Early trials |
| KRAS | G12D | Complete Match | MEK inhibitor;PI3K pathway inhibitor | Responsive | COREAD | Pre-clinical |
| KRAS | G12D | Complete Match | Cetuximab (EGFR mAb inhibitor) | Resistant | COREAD | NCCN guidelines |
| KRAS | G12D | Complete Match | Sorafenib;MEK inhibitor (Pan-TK inhibitor;MEK inhibitor) | Responsive | HC | Early trials |
| KRAS | G12D | Complete Match | EGFR inhibitor | Resistant | L | NCCN guidelines |
| KRAS | G12D | Complete Match | CDK4 inhibitor | Responsive | L | Pre-clinical |
| KRAS | G12D | Complete Match | Selumetinib (MEK inhibitor) | Responsive | NSCLC | Early trials |
| KRAS | G12D | Complete Match | BET inhibitor | Responsive | L | Pre-clinical |
| KRAS | G12D | Complete Match | Decitabine;BCL2 inhibitor (Chemotherapy;BCL2 inhibitor) | Responsive | OV | Pre-clinical |
| KRAS | G12D | Complete Match | FAK inhibitor | Responsive | L | Pre-clinical |
| KRAS | G12D | Complete Match | EGFR inhibitor | Resistant | NSCLC | Pre-clinical |
| KRAS | G12D | Complete Match | EZH2 inhibitor | Resistant | CANCER | Pre-clinical |
| KRAS | G12D | Complete Match | MEK inhibitor | Responsive | BT, L | Early trials |
| KRAS | G12D | Complete Match | MEK inhibitor;IGF1R inhibitor | Responsive | COREAD | Pre-clinical |
| KRAS | G12D | Complete Match | MTOR inhibitor;BH3 mimetics | Responsive | COREAD | Pre-clinical |
| KRAS | G12D | Complete Match | EGFR mAb inhibitor;MEK inhibitor | Responsive | COREAD | Pre-clinical |
| KRAS | G12D | Complete Match | pan-RAF inhibitor | Responsive | ED | Case report |
| KRAS | G12D | Complete Match | EGFR mAb inhibitor | Resistant | ST | Pre-clinical |
| KRAS | G12D | Complete Match | PI3K pathway inhibitor;MEK inhibitor | Responsive | L | Early trials |
| KRAS | G12D | Complete Match | Selumetinib (MEK inhibitor) | No Responsive | L | Early trials |
| KRAS | G12D | Complete Match | CDK4/6 inhibitor;MEK inhibitor | Responsive | L | Early trials |
| KRAS | G12D | Complete Match | Trametinib;Ponatinib (MEK inhibitor;BCR-ABL inhibitor & Pan-TK inhibitor) | Responsive | LUAD | Pre-clinical |
| KRAS | G12D | Complete Match | Trastuzumab;Lapatinib (ERBB2 mAb inhibitor;ERBB2 inhibitor) | Resistant | COREAD | Late trials |
| KRAS | G12D | Complete Match | pan-RAF inhibitor | Responsive | L | Early trials |
| KRAS | G12D | Complete Match | JAK/TBK1/IKKε inhibitor | Responsive | L | Pre-clinical |
| KRAS | G12D | Complete Match | FAS inhibitor | Responsive | L | Case report |
| KRAS | G12D | Complete Match | HSP90 inhibitor | Responsive | L | Pre-clinical |
| KRAS | G12D | Complete Match | ERK inhibitor | Responsive | COREAD | Pre-clinical |
| KRAS | G12D | Complete Match | PI3K pathway inhibitor;MEK inhibitor | No Responsive | PA | Early trials |
| KRAS | G12D | Complete Match | MEK inhibitor;BCL-XL inhibitor | Responsive | COREAD | Pre-clinical |
| KRAS | G12D | Complete Match | Gemcitabine;MEK inhibitor (Chemotherapy;MEK inhibitor) | Responsive | PA | Early trials |
| KRAS | G12D | Complete Match | Panitumumab (EGFR mAb inhibitor) | Resistant | COREAD | NCCN guidelines |
| KRAS | G12D | Complete Match | PI3K pathway inhibitor | Resistant | ED | Pre-clinical |
| KRAS | G12D | Complete Match | CDK4/6 inhibitor;MEK inhibitor | Responsive | CESC | Pre-clinical |
| KRAS | G12D | Different Alteration | BRAF inhibitor;MEK inhibitor | Resistant | COREAD | Case report |
| KRAS | G12D | Different Alteration | BRAF inhibitor;EGFR mAb inhibitor | Resistant | COREAD | Case report |
| KRAS | Q61L | Complete Match | Panitumumab (EGFR mAb inhibitor) | Resistant | COREAD | FDA guidelines |
| KRAS | Q61L | Different Mutation | Cetuximab (EGFR mAb inhibitor) | Resistant | COREAD | FDA guidelines |
| KRAS | Q61L | Different Mutation | Cetuximab (EGFR mAb inhibitor) | No Responsive | COREAD | Late trials |
| KRAS | Q61L | Different Mutation | MEK inhibitor | Responsive | ALL | Pre-clinical |
| KRAS | Q61L | Different Mutation | Imatinib (BCR-ABL inhibitor & KIT inhibitor) | Resistant | GIST | Case report |
| KRAS | Q61L | Complete Match | MEK inhibitor | Responsive | OV, CER, AML | Pre-clinical |
| KRAS | Q61L | Complete Match | Abemaciclib (CDK4/6 inhibitor) | Responsive | L | Early trials |
| KRAS | Q61L | Complete Match | MEK inhibitor;PI3K pathway inhibitor | Responsive | COREAD | Pre-clinical |
| KRAS | Q61L | Complete Match | Cetuximab (EGFR mAb inhibitor) | Resistant | COREAD | NCCN guidelines |
| KRAS | Q61L | Complete Match | Sorafenib;MEK inhibitor (Pan-TK inhibitor;MEK inhibitor) | Responsive | HC | Early trials |
| KRAS | Q61L | Complete Match | EGFR inhibitor | Resistant | L | NCCN guidelines |
| KRAS | Q61L | Complete Match | CDK4 inhibitor | Responsive | L | Pre-clinical |
| KRAS | Q61L | Complete Match | Selumetinib (MEK inhibitor) | Responsive | NSCLC | Early trials |
| KRAS | Q61L | Complete Match | BET inhibitor | Responsive | L | Pre-clinical |
| KRAS | Q61L | Complete Match | Decitabine;BCL2 inhibitor (Chemotherapy;BCL2 inhibitor) | Responsive | OV | Pre-clinical |
| KRAS | Q61L | Complete Match | FAK inhibitor | Responsive | L | Pre-clinical |
| KRAS | Q61L | Complete Match | EGFR inhibitor | Resistant | NSCLC | Pre-clinical |
| KRAS | Q61L | Complete Match | EZH2 inhibitor | Resistant | CANCER | Pre-clinical |
| KRAS | Q61L | Complete Match | MEK inhibitor | Responsive | BT, L | Early trials |
| KRAS | Q61L | Complete Match | MEK inhibitor;IGF1R inhibitor | Responsive | COREAD | Pre-clinical |
| KRAS | Q61L | Complete Match | MTOR inhibitor;BH3 mimetics | Responsive | COREAD | Pre-clinical |
| KRAS | Q61L | Complete Match | EGFR mAb inhibitor;MEK inhibitor | Responsive | COREAD | Pre-clinical |
| KRAS | Q61L | Complete Match | pan-RAF inhibitor | Responsive | ED | Case report |
| KRAS | Q61L | Complete Match | EGFR mAb inhibitor | Resistant | ST | Pre-clinical |
| KRAS | Q61L | Complete Match | PI3K pathway inhibitor;MEK inhibitor | Responsive | L | Early trials |
| KRAS | Q61L | Complete Match | Selumetinib (MEK inhibitor) | No Responsive | L | Early trials |
| KRAS | Q61L | Complete Match | CDK4/6 inhibitor;MEK inhibitor | Responsive | L | Early trials |
| KRAS | Q61L | Complete Match | Trametinib;Ponatinib (MEK inhibitor;BCR-ABL inhibitor & Pan-TK inhibitor) | Responsive | LUAD | Pre-clinical |
| KRAS | Q61L | Complete Match | Trastuzumab;Lapatinib (ERBB2 mAb inhibitor;ERBB2 inhibitor) | Resistant | COREAD | Late trials |
| KRAS | Q61L | Complete Match | pan-RAF inhibitor | Responsive | L | Early trials |
| KRAS | Q61L | Complete Match | JAK/TBK1/IKKε inhibitor | Responsive | L | Pre-clinical |
| KRAS | Q61L | Complete Match | FAS inhibitor | Responsive | L | Case report |
| KRAS | Q61L | Complete Match | HSP90 inhibitor | Responsive | L | Pre-clinical |
| KRAS | Q61L | Complete Match | ERK inhibitor | Responsive | COREAD | Pre-clinical |
| KRAS | Q61L | Complete Match | PI3K pathway inhibitor;MEK inhibitor | No Responsive | PA | Early trials |
| KRAS | Q61L | Complete Match | MEK inhibitor;BCL-XL inhibitor | Responsive | COREAD | Pre-clinical |
| KRAS | Q61L | Complete Match | Gemcitabine;MEK inhibitor (Chemotherapy;MEK inhibitor) | Responsive | PA | Early trials |
| KRAS | Q61L | Complete Match | Panitumumab (EGFR mAb inhibitor) | Resistant | COREAD | NCCN guidelines |
| KRAS | Q61L | Complete Match | PI3K pathway inhibitor | Resistant | ED | Pre-clinical |
| KRAS | Q61L | Complete Match | CDK4/6 inhibitor;MEK inhibitor | Responsive | CESC | Pre-clinical |
| KRAS | Q61L | Different Alteration | BRAF inhibitor;MEK inhibitor | Resistant | COREAD | Case report |
| KRAS | Q61L | Different Alteration | BRAF inhibitor;EGFR mAb inhibitor | Resistant | COREAD | Case report |
| KRAS | G12V | Complete Match | Panitumumab (EGFR mAb inhibitor) | Resistant | COREAD | FDA guidelines |
| KRAS | G12V | Complete Match | Cetuximab (EGFR mAb inhibitor) | Resistant | COREAD | FDA guidelines |
| KRAS | G12V | Different Mutation | Cetuximab (EGFR mAb inhibitor) | No Responsive | COREAD | Late trials |
| KRAS | G12V | Complete Match | MEK inhibitor | Responsive | ALL | Pre-clinical |
| KRAS | G12V | Complete Match | Imatinib (BCR-ABL inhibitor & KIT inhibitor) | Resistant | GIST | Case report |
| KRAS | G12V | Complete Match | MEK inhibitor | Responsive | OV, CER, AML | Pre-clinical |
| KRAS | G12V | Complete Match | Abemaciclib (CDK4/6 inhibitor) | Responsive | L | Early trials |
| KRAS | G12V | Complete Match | MEK inhibitor;PI3K pathway inhibitor | Responsive | COREAD | Pre-clinical |
| KRAS | G12V | Complete Match | Cetuximab (EGFR mAb inhibitor) | Resistant | COREAD | NCCN guidelines |
| KRAS | G12V | Complete Match | Sorafenib;MEK inhibitor (Pan-TK inhibitor;MEK inhibitor) | Responsive | HC | Early trials |
| KRAS | G12V | Complete Match | EGFR inhibitor | Resistant | L | NCCN guidelines |
| KRAS | G12V | Complete Match | CDK4 inhibitor | Responsive | L | Pre-clinical |
| KRAS | G12V | Complete Match | Selumetinib (MEK inhibitor) | Responsive | NSCLC | Early trials |
| KRAS | G12V | Complete Match | BET inhibitor | Responsive | L | Pre-clinical |
| KRAS | G12V | Complete Match | Decitabine;BCL2 inhibitor (Chemotherapy;BCL2 inhibitor) | Responsive | OV | Pre-clinical |
| KRAS | G12V | Complete Match | FAK inhibitor | Responsive | L | Pre-clinical |
| KRAS | G12V | Complete Match | EGFR inhibitor | Resistant | NSCLC | Pre-clinical |
| KRAS | G12V | Complete Match | EZH2 inhibitor | Resistant | CANCER | Pre-clinical |
| KRAS | G12V | Complete Match | MEK inhibitor | Responsive | BT, L | Early trials |
| KRAS | G12V | Complete Match | MEK inhibitor;IGF1R inhibitor | Responsive | COREAD | Pre-clinical |
| KRAS | G12V | Complete Match | MTOR inhibitor;BH3 mimetics | Responsive | COREAD | Pre-clinical |
| KRAS | G12V | Complete Match | EGFR mAb inhibitor;MEK inhibitor | Responsive | COREAD | Pre-clinical |
| KRAS | G12V | Complete Match | pan-RAF inhibitor | Responsive | ED | Case report |
| KRAS | G12V | Complete Match | EGFR mAb inhibitor | Resistant | ST | Pre-clinical |
| KRAS | G12V | Complete Match | PI3K pathway inhibitor;MEK inhibitor | Responsive | L | Early trials |
| KRAS | G12V | Complete Match | Selumetinib (MEK inhibitor) | No Responsive | L | Early trials |
| KRAS | G12V | Complete Match | CDK4/6 inhibitor;MEK inhibitor | Responsive | L | Early trials |
| KRAS | G12V | Complete Match | Trametinib;Ponatinib (MEK inhibitor;BCR-ABL inhibitor & Pan-TK inhibitor) | Responsive | LUAD | Pre-clinical |
| KRAS | G12V | Complete Match | Trastuzumab;Lapatinib (ERBB2 mAb inhibitor;ERBB2 inhibitor) | Resistant | COREAD | Late trials |
| KRAS | G12V | Complete Match | pan-RAF inhibitor | Responsive | L | Early trials |
| KRAS | G12V | Complete Match | JAK/TBK1/IKKε inhibitor | Responsive | L | Pre-clinical |
| KRAS | G12V | Complete Match | FAS inhibitor | Responsive | L | Case report |
| KRAS | G12V | Complete Match | HSP90 inhibitor | Responsive | L | Pre-clinical |
| KRAS | G12V | Complete Match | ERK inhibitor | Responsive | COREAD | Pre-clinical |
| KRAS | G12V | Complete Match | PI3K pathway inhibitor;MEK inhibitor | No Responsive | PA | Early trials |
| KRAS | G12V | Complete Match | MEK inhibitor;BCL-XL inhibitor | Responsive | COREAD | Pre-clinical |
| KRAS | G12V | Complete Match | Gemcitabine;MEK inhibitor (Chemotherapy;MEK inhibitor) | Responsive | PA | Early trials |
| KRAS | G12V | Complete Match | Panitumumab (EGFR mAb inhibitor) | Resistant | COREAD | NCCN guidelines |
| KRAS | G12V | Complete Match | PI3K pathway inhibitor | Resistant | ED | Pre-clinical |
| KRAS | G12V | Complete Match | CDK4/6 inhibitor;MEK inhibitor | Responsive | CESC | Pre-clinical |
| KRAS | G12V | Different Alteration | BRAF inhibitor;MEK inhibitor | Resistant | COREAD | Case report |
| KRAS | G12V | Different Alteration | BRAF inhibitor;EGFR mAb inhibitor | Resistant | COREAD | Case report |
| KRAS | Q61H | Complete Match | Panitumumab (EGFR mAb inhibitor) | Resistant | COREAD | FDA guidelines |
| KRAS | Q61H | Different Mutation | Cetuximab (EGFR mAb inhibitor) | Resistant | COREAD | FDA guidelines |
| KRAS | Q61H | Different Mutation | Cetuximab (EGFR mAb inhibitor) | No Responsive | COREAD | Late trials |
| KRAS | Q61H | Different Mutation | MEK inhibitor | Responsive | ALL | Pre-clinical |
| KRAS | Q61H | Different Mutation | Imatinib (BCR-ABL inhibitor & KIT inhibitor) | Resistant | GIST | Case report |
| KRAS | Q61H | Complete Match | MEK inhibitor | Responsive | OV, CER, AML | Pre-clinical |
| KRAS | Q61H | Complete Match | Abemaciclib (CDK4/6 inhibitor) | Responsive | L | Early trials |
| KRAS | Q61H | Complete Match | MEK inhibitor;PI3K pathway inhibitor | Responsive | COREAD | Pre-clinical |
| KRAS | Q61H | Complete Match | Cetuximab (EGFR mAb inhibitor) | Resistant | COREAD | NCCN guidelines |
| KRAS | Q61H | Complete Match | Sorafenib;MEK inhibitor (Pan-TK inhibitor;MEK inhibitor) | Responsive | HC | Early trials |
| KRAS | Q61H | Complete Match | EGFR inhibitor | Resistant | L | NCCN guidelines |
| KRAS | Q61H | Complete Match | CDK4 inhibitor | Responsive | L | Pre-clinical |
| KRAS | Q61H | Complete Match | Selumetinib (MEK inhibitor) | Responsive | NSCLC | Early trials |
| KRAS | Q61H | Complete Match | BET inhibitor | Responsive | L | Pre-clinical |
| KRAS | Q61H | Complete Match | Decitabine;BCL2 inhibitor (Chemotherapy;BCL2 inhibitor) | Responsive | OV | Pre-clinical |
| KRAS | Q61H | Complete Match | FAK inhibitor | Responsive | L | Pre-clinical |
| KRAS | Q61H | Complete Match | EGFR inhibitor | Resistant | NSCLC | Pre-clinical |
| KRAS | Q61H | Complete Match | EZH2 inhibitor | Resistant | CANCER | Pre-clinical |
| KRAS | Q61H | Complete Match | MEK inhibitor | Responsive | BT, L | Early trials |
| KRAS | Q61H | Complete Match | MEK inhibitor;IGF1R inhibitor | Responsive | COREAD | Pre-clinical |
| KRAS | Q61H | Complete Match | MTOR inhibitor;BH3 mimetics | Responsive | COREAD | Pre-clinical |
| KRAS | Q61H | Complete Match | EGFR mAb inhibitor;MEK inhibitor | Responsive | COREAD | Pre-clinical |
| KRAS | Q61H | Complete Match | pan-RAF inhibitor | Responsive | ED | Case report |
| KRAS | Q61H | Complete Match | EGFR mAb inhibitor | Resistant | ST | Pre-clinical |
| KRAS | Q61H | Complete Match | PI3K pathway inhibitor;MEK inhibitor | Responsive | L | Early trials |
| KRAS | Q61H | Complete Match | Selumetinib (MEK inhibitor) | No Responsive | L | Early trials |
| KRAS | Q61H | Complete Match | CDK4/6 inhibitor;MEK inhibitor | Responsive | L | Early trials |
| KRAS | Q61H | Complete Match | Trametinib;Ponatinib (MEK inhibitor;BCR-ABL inhibitor & Pan-TK inhibitor) | Responsive | LUAD | Pre-clinical |
| KRAS | Q61H | Complete Match | Trastuzumab;Lapatinib (ERBB2 mAb inhibitor;ERBB2 inhibitor) | Resistant | COREAD | Late trials |
| KRAS | Q61H | Complete Match | pan-RAF inhibitor | Responsive | L | Early trials |
| KRAS | Q61H | Complete Match | JAK/TBK1/IKKε inhibitor | Responsive | L | Pre-clinical |
| KRAS | Q61H | Complete Match | FAS inhibitor | Responsive | L | Case report |
| KRAS | Q61H | Complete Match | HSP90 inhibitor | Responsive | L | Pre-clinical |
| KRAS | Q61H | Complete Match | ERK inhibitor | Responsive | COREAD | Pre-clinical |
| KRAS | Q61H | Complete Match | PI3K pathway inhibitor;MEK inhibitor | No Responsive | PA | Early trials |
| KRAS | Q61H | Complete Match | MEK inhibitor;BCL-XL inhibitor | Responsive | COREAD | Pre-clinical |
| KRAS | Q61H | Complete Match | Gemcitabine;MEK inhibitor (Chemotherapy;MEK inhibitor) | Responsive | PA | Early trials |
| KRAS | Q61H | Complete Match | Panitumumab (EGFR mAb inhibitor) | Resistant | COREAD | NCCN guidelines |
| KRAS | Q61H | Complete Match | PI3K pathway inhibitor | Resistant | ED | Pre-clinical |
| KRAS | Q61H | Complete Match | CDK4/6 inhibitor;MEK inhibitor | Responsive | CESC | Pre-clinical |
| KRAS | Q61H | Different Alteration | BRAF inhibitor;MEK inhibitor | Resistant | COREAD | Case report |
| KRAS | Q61H | Different Alteration | BRAF inhibitor;EGFR mAb inhibitor | Resistant | COREAD | Case report |
| KRAS | G12R), KRAS MUT (G12D | Complete Match | Panitumumab (EGFR mAb inhibitor) | Resistant | COREAD | FDA guidelines |
| KRAS | G12R), KRAS MUT (G12D | Complete Match | Cetuximab (EGFR mAb inhibitor) | Resistant | COREAD | FDA guidelines |
| KRAS | G12R), KRAS MUT (G12D | Different Mutation | Cetuximab (EGFR mAb inhibitor) | No Responsive | COREAD | Late trials |
| KRAS | G12R), KRAS MUT (G12D | Complete Match | MEK inhibitor | Responsive | ALL | Pre-clinical |
| KRAS | G12R), KRAS MUT (G12D | Complete Match | Imatinib (BCR-ABL inhibitor & KIT inhibitor) | Resistant | GIST | Case report |
| KRAS | G12R), KRAS MUT (G12D | Complete Match | MEK inhibitor | Responsive | OV, CER, AML | Pre-clinical |
| KRAS | G12R), KRAS MUT (G12D | Complete Match | Abemaciclib (CDK4/6 inhibitor) | Responsive | L | Early trials |
| KRAS | G12R), KRAS MUT (G12D | Complete Match | MEK inhibitor;PI3K pathway inhibitor | Responsive | COREAD | Pre-clinical |
| KRAS | G12R), KRAS MUT (G12D | Complete Match | Cetuximab (EGFR mAb inhibitor) | Resistant | COREAD | NCCN guidelines |
| KRAS | G12R), KRAS MUT (G12D | Complete Match | Sorafenib;MEK inhibitor (Pan-TK inhibitor;MEK inhibitor) | Responsive | HC | Early trials |
| KRAS | G12R), KRAS MUT (G12D | Complete Match | EGFR inhibitor | Resistant | L | NCCN guidelines |
| KRAS | G12R), KRAS MUT (G12D | Complete Match | CDK4 inhibitor | Responsive | L | Pre-clinical |
| KRAS | G12R), KRAS MUT (G12D | Complete Match | Selumetinib (MEK inhibitor) | Responsive | NSCLC | Early trials |
| KRAS | G12R), KRAS MUT (G12D | Complete Match | BET inhibitor | Responsive | L | Pre-clinical |
| KRAS | G12R), KRAS MUT (G12D | Complete Match | Decitabine;BCL2 inhibitor (Chemotherapy;BCL2 inhibitor) | Responsive | OV | Pre-clinical |
| KRAS | G12R), KRAS MUT (G12D | Complete Match | FAK inhibitor | Responsive | L | Pre-clinical |
| KRAS | G12R), KRAS MUT (G12D | Complete Match | EGFR inhibitor | Resistant | NSCLC | Pre-clinical |
| KRAS | G12R), KRAS MUT (G12D | Complete Match | EZH2 inhibitor | Resistant | CANCER | Pre-clinical |
| KRAS | G12R), KRAS MUT (G12D | Complete Match | MEK inhibitor | Responsive | BT, L | Early trials |
| KRAS | G12R), KRAS MUT (G12D | Complete Match | MEK inhibitor;IGF1R inhibitor | Responsive | COREAD | Pre-clinical |
| KRAS | G12R), KRAS MUT (G12D | Complete Match | MTOR inhibitor;BH3 mimetics | Responsive | COREAD | Pre-clinical |
| KRAS | G12R), KRAS MUT (G12D | Complete Match | EGFR mAb inhibitor;MEK inhibitor | Responsive | COREAD | Pre-clinical |
| KRAS | G12R), KRAS MUT (G12D | Complete Match | pan-RAF inhibitor | Responsive | ED | Case report |
| KRAS | G12R), KRAS MUT (G12D | Complete Match | EGFR mAb inhibitor | Resistant | ST | Pre-clinical |
| KRAS | G12R), KRAS MUT (G12D | Complete Match | PI3K pathway inhibitor;MEK inhibitor | Responsive | L | Early trials |
| KRAS | G12R), KRAS MUT (G12D | Complete Match | Selumetinib (MEK inhibitor) | No Responsive | L | Early trials |
| KRAS | G12R), KRAS MUT (G12D | Complete Match | CDK4/6 inhibitor;MEK inhibitor | Responsive | L | Early trials |
| KRAS | G12R), KRAS MUT (G12D | Complete Match | Trametinib;Ponatinib (MEK inhibitor;BCR-ABL inhibitor & Pan-TK inhibitor) | Responsive | LUAD | Pre-clinical |
| KRAS | G12R), KRAS MUT (G12D | Complete Match | Trastuzumab;Lapatinib (ERBB2 mAb inhibitor;ERBB2 inhibitor) | Resistant | COREAD | Late trials |
| KRAS | G12R), KRAS MUT (G12D | Complete Match | pan-RAF inhibitor | Responsive | L | Early trials |
| KRAS | G12R), KRAS MUT (G12D | Complete Match | JAK/TBK1/IKKε inhibitor | Responsive | L | Pre-clinical |
| KRAS | G12R), KRAS MUT (G12D | Complete Match | FAS inhibitor | Responsive | L | Case report |
| KRAS | G12R), KRAS MUT (G12D | Complete Match | HSP90 inhibitor | Responsive | L | Pre-clinical |
| KRAS | G12R), KRAS MUT (G12D | Complete Match | ERK inhibitor | Responsive | COREAD | Pre-clinical |
| KRAS | G12R), KRAS MUT (G12D | Complete Match | PI3K pathway inhibitor;MEK inhibitor | No Responsive | PA | Early trials |
| KRAS | G12R), KRAS MUT (G12D | Complete Match | MEK inhibitor;BCL-XL inhibitor | Responsive | COREAD | Pre-clinical |
| KRAS | G12R), KRAS MUT (G12D | Complete Match | Gemcitabine;MEK inhibitor (Chemotherapy;MEK inhibitor) | Responsive | PA | Early trials |
| KRAS | G12R), KRAS MUT (G12D | Complete Match | Panitumumab (EGFR mAb inhibitor) | Resistant | COREAD | NCCN guidelines |
| KRAS | G12R), KRAS MUT (G12D | Complete Match | PI3K pathway inhibitor | Resistant | ED | Pre-clinical |
| KRAS | G12R), KRAS MUT (G12D | Complete Match | CDK4/6 inhibitor;MEK inhibitor | Responsive | CESC | Pre-clinical |
| KRAS | G12R), KRAS MUT (G12D | Different Alteration | BRAF inhibitor;MEK inhibitor | Resistant | COREAD | Case report |
| KRAS | G12R), KRAS MUT (G12D | Different Alteration | BRAF inhibitor;EGFR mAb inhibitor | Resistant | COREAD | Case report |
| KRAS | Q61R | Complete Match | Panitumumab (EGFR mAb inhibitor) | Resistant | COREAD | FDA guidelines |
| KRAS | Q61R | Different Mutation | Cetuximab (EGFR mAb inhibitor) | Resistant | COREAD | FDA guidelines |
| KRAS | Q61R | Different Mutation | Cetuximab (EGFR mAb inhibitor) | No Responsive | COREAD | Late trials |
| KRAS | Q61R | Different Mutation | MEK inhibitor | Responsive | ALL | Pre-clinical |
| KRAS | Q61R | Different Mutation | Imatinib (BCR-ABL inhibitor & KIT inhibitor) | Resistant | GIST | Case report |
| KRAS | Q61R | Complete Match | MEK inhibitor | Responsive | OV, CER, AML | Pre-clinical |
| KRAS | Q61R | Complete Match | Abemaciclib (CDK4/6 inhibitor) | Responsive | L | Early trials |
| KRAS | Q61R | Complete Match | MEK inhibitor;PI3K pathway inhibitor | Responsive | COREAD | Pre-clinical |
| KRAS | Q61R | Complete Match | Cetuximab (EGFR mAb inhibitor) | Resistant | COREAD | NCCN guidelines |
| KRAS | Q61R | Complete Match | Sorafenib;MEK inhibitor (Pan-TK inhibitor;MEK inhibitor) | Responsive | HC | Early trials |
| KRAS | Q61R | Complete Match | EGFR inhibitor | Resistant | L | NCCN guidelines |
| KRAS | Q61R | Complete Match | CDK4 inhibitor | Responsive | L | Pre-clinical |
| KRAS | Q61R | Complete Match | Selumetinib (MEK inhibitor) | Responsive | NSCLC | Early trials |
| KRAS | Q61R | Complete Match | BET inhibitor | Responsive | L | Pre-clinical |
| KRAS | Q61R | Complete Match | Decitabine;BCL2 inhibitor (Chemotherapy;BCL2 inhibitor) | Responsive | OV | Pre-clinical |
| KRAS | Q61R | Complete Match | FAK inhibitor | Responsive | L | Pre-clinical |
| KRAS | Q61R | Complete Match | EGFR inhibitor | Resistant | NSCLC | Pre-clinical |
| KRAS | Q61R | Complete Match | EZH2 inhibitor | Resistant | CANCER | Pre-clinical |
| KRAS | Q61R | Complete Match | MEK inhibitor | Responsive | BT, L | Early trials |
| KRAS | Q61R | Complete Match | MEK inhibitor;IGF1R inhibitor | Responsive | COREAD | Pre-clinical |
| KRAS | Q61R | Complete Match | MTOR inhibitor;BH3 mimetics | Responsive | COREAD | Pre-clinical |
| KRAS | Q61R | Complete Match | EGFR mAb inhibitor;MEK inhibitor | Responsive | COREAD | Pre-clinical |
| KRAS | Q61R | Complete Match | pan-RAF inhibitor | Responsive | ED | Case report |
| KRAS | Q61R | Complete Match | EGFR mAb inhibitor | Resistant | ST | Pre-clinical |
| KRAS | Q61R | Complete Match | PI3K pathway inhibitor;MEK inhibitor | Responsive | L | Early trials |
| KRAS | Q61R | Complete Match | Selumetinib (MEK inhibitor) | No Responsive | L | Early trials |
| KRAS | Q61R | Complete Match | CDK4/6 inhibitor;MEK inhibitor | Responsive | L | Early trials |
| KRAS | Q61R | Complete Match | Trametinib;Ponatinib (MEK inhibitor;BCR-ABL inhibitor & Pan-TK inhibitor) | Responsive | LUAD | Pre-clinical |
| KRAS | Q61R | Complete Match | Trastuzumab;Lapatinib (ERBB2 mAb inhibitor;ERBB2 inhibitor) | Resistant | COREAD | Late trials |
| KRAS | Q61R | Complete Match | pan-RAF inhibitor | Responsive | L | Early trials |
| KRAS | Q61R | Complete Match | JAK/TBK1/IKKε inhibitor | Responsive | L | Pre-clinical |
| KRAS | Q61R | Complete Match | FAS inhibitor | Responsive | L | Case report |
| KRAS | Q61R | Complete Match | HSP90 inhibitor | Responsive | L | Pre-clinical |
| KRAS | Q61R | Complete Match | ERK inhibitor | Responsive | COREAD | Pre-clinical |
| KRAS | Q61R | Complete Match | PI3K pathway inhibitor;MEK inhibitor | No Responsive | PA | Early trials |
| KRAS | Q61R | Complete Match | MEK inhibitor;BCL-XL inhibitor | Responsive | COREAD | Pre-clinical |
| KRAS | Q61R | Complete Match | Gemcitabine;MEK inhibitor (Chemotherapy;MEK inhibitor) | Responsive | PA | Early trials |
| KRAS | Q61R | Complete Match | Panitumumab (EGFR mAb inhibitor) | Resistant | COREAD | NCCN guidelines |
| KRAS | Q61R | Complete Match | PI3K pathway inhibitor | Resistant | ED | Pre-clinical |
| KRAS | Q61R | Complete Match | CDK4/6 inhibitor;MEK inhibitor | Responsive | CESC | Pre-clinical |
| KRAS | Q61R | Different Alteration | BRAF inhibitor;MEK inhibitor | Resistant | COREAD | Case report |
| KRAS | Q61R | Different Alteration | BRAF inhibitor;EGFR mAb inhibitor | Resistant | COREAD | Case report |
| KRAS | G12C | Complete Match | Panitumumab (EGFR mAb inhibitor) | Resistant | COREAD | FDA guidelines |
| KRAS | G12C | Complete Match | Cetuximab (EGFR mAb inhibitor) | Resistant | COREAD | FDA guidelines |
| KRAS | G12C | Different Mutation | Cetuximab (EGFR mAb inhibitor) | No Responsive | COREAD | Late trials |
| KRAS | G12C | Complete Match | MEK inhibitor | Responsive | ALL | Pre-clinical |
| KRAS | G12C | Complete Match | Imatinib (BCR-ABL inhibitor & KIT inhibitor) | Resistant | GIST | Case report |
| KRAS | G12C | Complete Match | MEK inhibitor | Responsive | OV, CER, AML | Pre-clinical |
| KRAS | G12C | Complete Match | Abemaciclib (CDK4/6 inhibitor) | Responsive | L | Early trials |
| KRAS | G12C | Complete Match | MEK inhibitor;PI3K pathway inhibitor | Responsive | COREAD | Pre-clinical |
| KRAS | G12C | Complete Match | Cetuximab (EGFR mAb inhibitor) | Resistant | COREAD | NCCN guidelines |
| KRAS | G12C | Complete Match | Sorafenib;MEK inhibitor (Pan-TK inhibitor;MEK inhibitor) | Responsive | HC | Early trials |
| KRAS | G12C | Complete Match | EGFR inhibitor | Resistant | L | NCCN guidelines |
| KRAS | G12C | Complete Match | CDK4 inhibitor | Responsive | L | Pre-clinical |
| KRAS | G12C | Complete Match | Selumetinib (MEK inhibitor) | Responsive | NSCLC | Early trials |
| KRAS | G12C | Complete Match | BET inhibitor | Responsive | L | Pre-clinical |
| KRAS | G12C | Complete Match | Decitabine;BCL2 inhibitor (Chemotherapy;BCL2 inhibitor) | Responsive | OV | Pre-clinical |
| KRAS | G12C | Complete Match | FAK inhibitor | Responsive | L | Pre-clinical |
| KRAS | G12C | Complete Match | EGFR inhibitor | Resistant | NSCLC | Pre-clinical |
| KRAS | G12C | Complete Match | EZH2 inhibitor | Resistant | CANCER | Pre-clinical |
| KRAS | G12C | Complete Match | MEK inhibitor | Responsive | BT, L | Early trials |
| KRAS | G12C | Complete Match | MEK inhibitor;IGF1R inhibitor | Responsive | COREAD | Pre-clinical |
| KRAS | G12C | Complete Match | MTOR inhibitor;BH3 mimetics | Responsive | COREAD | Pre-clinical |
| KRAS | G12C | Complete Match | EGFR mAb inhibitor;MEK inhibitor | Responsive | COREAD | Pre-clinical |
| KRAS | G12C | Complete Match | pan-RAF inhibitor | Responsive | ED | Case report |
| KRAS | G12C | Complete Match | EGFR mAb inhibitor | Resistant | ST | Pre-clinical |
| KRAS | G12C | Complete Match | PI3K pathway inhibitor;MEK inhibitor | Responsive | L | Early trials |
| KRAS | G12C | Complete Match | Selumetinib (MEK inhibitor) | No Responsive | L | Early trials |
| KRAS | G12C | Complete Match | CDK4/6 inhibitor;MEK inhibitor | Responsive | L | Early trials |
| KRAS | G12C | Complete Match | Trametinib;Ponatinib (MEK inhibitor;BCR-ABL inhibitor & Pan-TK inhibitor) | Responsive | LUAD | Pre-clinical |
| KRAS | G12C | Complete Match | Trastuzumab;Lapatinib (ERBB2 mAb inhibitor;ERBB2 inhibitor) | Resistant | COREAD | Late trials |
| KRAS | G12C | Complete Match | pan-RAF inhibitor | Responsive | L | Early trials |
| KRAS | G12C | Complete Match | JAK/TBK1/IKKε inhibitor | Responsive | L | Pre-clinical |
| KRAS | G12C | Complete Match | FAS inhibitor | Responsive | L | Case report |
| KRAS | G12C | Complete Match | HSP90 inhibitor | Responsive | L | Pre-clinical |
| KRAS | G12C | Complete Match | ERK inhibitor | Responsive | COREAD | Pre-clinical |
| KRAS | G12C | Complete Match | PI3K pathway inhibitor;MEK inhibitor | No Responsive | PA | Early trials |
| KRAS | G12C | Complete Match | MEK inhibitor;BCL-XL inhibitor | Responsive | COREAD | Pre-clinical |
| KRAS | G12C | Complete Match | Gemcitabine;MEK inhibitor (Chemotherapy;MEK inhibitor) | Responsive | PA | Early trials |
| KRAS | G12C | Complete Match | Panitumumab (EGFR mAb inhibitor) | Resistant | COREAD | NCCN guidelines |
| KRAS | G12C | Complete Match | PI3K pathway inhibitor | Resistant | ED | Pre-clinical |
| KRAS | G12C | Complete Match | CDK4/6 inhibitor;MEK inhibitor | Responsive | CESC | Pre-clinical |
| KRAS | G12C | Different Alteration | BRAF inhibitor;MEK inhibitor | Resistant | COREAD | Case report |
| KRAS | G12C | Different Alteration | BRAF inhibitor;EGFR mAb inhibitor | Resistant | COREAD | Case report |
| KRAS | G12D), KRAS MUT (G12V | Complete Match | Panitumumab (EGFR mAb inhibitor) | Resistant | COREAD | FDA guidelines |
| KRAS | G12D), KRAS MUT (G12V | Complete Match | Cetuximab (EGFR mAb inhibitor) | Resistant | COREAD | FDA guidelines |
| KRAS | G12D), KRAS MUT (G12V | Different Mutation | Cetuximab (EGFR mAb inhibitor) | No Responsive | COREAD | Late trials |
| KRAS | G12D), KRAS MUT (G12V | Complete Match | MEK inhibitor | Responsive | ALL | Pre-clinical |
| KRAS | G12D), KRAS MUT (G12V | Complete Match | Imatinib (BCR-ABL inhibitor & KIT inhibitor) | Resistant | GIST | Case report |
| KRAS | G12D), KRAS MUT (G12V | Complete Match | MEK inhibitor | Responsive | OV, CER, AML | Pre-clinical |
| KRAS | G12D), KRAS MUT (G12V | Complete Match | Abemaciclib (CDK4/6 inhibitor) | Responsive | L | Early trials |
| KRAS | G12D), KRAS MUT (G12V | Complete Match | MEK inhibitor;PI3K pathway inhibitor | Responsive | COREAD | Pre-clinical |
| KRAS | G12D), KRAS MUT (G12V | Complete Match | Cetuximab (EGFR mAb inhibitor) | Resistant | COREAD | NCCN guidelines |
| KRAS | G12D), KRAS MUT (G12V | Complete Match | Sorafenib;MEK inhibitor (Pan-TK inhibitor;MEK inhibitor) | Responsive | HC | Early trials |
| KRAS | G12D), KRAS MUT (G12V | Complete Match | EGFR inhibitor | Resistant | L | NCCN guidelines |
| KRAS | G12D), KRAS MUT (G12V | Complete Match | CDK4 inhibitor | Responsive | L | Pre-clinical |
| KRAS | G12D), KRAS MUT (G12V | Complete Match | Selumetinib (MEK inhibitor) | Responsive | NSCLC | Early trials |
| KRAS | G12D), KRAS MUT (G12V | Complete Match | BET inhibitor | Responsive | L | Pre-clinical |
| KRAS | G12D), KRAS MUT (G12V | Complete Match | Decitabine;BCL2 inhibitor (Chemotherapy;BCL2 inhibitor) | Responsive | OV | Pre-clinical |
| KRAS | G12D), KRAS MUT (G12V | Complete Match | FAK inhibitor | Responsive | L | Pre-clinical |
| KRAS | G12D), KRAS MUT (G12V | Complete Match | EGFR inhibitor | Resistant | NSCLC | Pre-clinical |
| KRAS | G12D), KRAS MUT (G12V | Complete Match | EZH2 inhibitor | Resistant | CANCER | Pre-clinical |
| KRAS | G12D), KRAS MUT (G12V | Complete Match | MEK inhibitor | Responsive | BT, L | Early trials |
| KRAS | G12D), KRAS MUT (G12V | Complete Match | MEK inhibitor;IGF1R inhibitor | Responsive | COREAD | Pre-clinical |
| KRAS | G12D), KRAS MUT (G12V | Complete Match | MTOR inhibitor;BH3 mimetics | Responsive | COREAD | Pre-clinical |
| KRAS | G12D), KRAS MUT (G12V | Complete Match | EGFR mAb inhibitor;MEK inhibitor | Responsive | COREAD | Pre-clinical |
| KRAS | G12D), KRAS MUT (G12V | Complete Match | pan-RAF inhibitor | Responsive | ED | Case report |
| KRAS | G12D), KRAS MUT (G12V | Complete Match | EGFR mAb inhibitor | Resistant | ST | Pre-clinical |
| KRAS | G12D), KRAS MUT (G12V | Complete Match | PI3K pathway inhibitor;MEK inhibitor | Responsive | L | Early trials |
| KRAS | G12D), KRAS MUT (G12V | Complete Match | Selumetinib (MEK inhibitor) | No Responsive | L | Early trials |
| KRAS | G12D), KRAS MUT (G12V | Complete Match | CDK4/6 inhibitor;MEK inhibitor | Responsive | L | Early trials |
| KRAS | G12D), KRAS MUT (G12V | Complete Match | Trametinib;Ponatinib (MEK inhibitor;BCR-ABL inhibitor & Pan-TK inhibitor) | Responsive | LUAD | Pre-clinical |
| KRAS | G12D), KRAS MUT (G12V | Complete Match | Trastuzumab;Lapatinib (ERBB2 mAb inhibitor;ERBB2 inhibitor) | Resistant | COREAD | Late trials |
| KRAS | G12D), KRAS MUT (G12V | Complete Match | pan-RAF inhibitor | Responsive | L | Early trials |
| KRAS | G12D), KRAS MUT (G12V | Complete Match | JAK/TBK1/IKKε inhibitor | Responsive | L | Pre-clinical |
| KRAS | G12D), KRAS MUT (G12V | Complete Match | FAS inhibitor | Responsive | L | Case report |
| KRAS | G12D), KRAS MUT (G12V | Complete Match | HSP90 inhibitor | Responsive | L | Pre-clinical |
| KRAS | G12D), KRAS MUT (G12V | Complete Match | ERK inhibitor | Responsive | COREAD | Pre-clinical |
| KRAS | G12D), KRAS MUT (G12V | Complete Match | PI3K pathway inhibitor;MEK inhibitor | No Responsive | PA | Early trials |
| KRAS | G12D), KRAS MUT (G12V | Complete Match | MEK inhibitor;BCL-XL inhibitor | Responsive | COREAD | Pre-clinical |
| KRAS | G12D), KRAS MUT (G12V | Complete Match | Gemcitabine;MEK inhibitor (Chemotherapy;MEK inhibitor) | Responsive | PA | Early trials |
| KRAS | G12D), KRAS MUT (G12V | Complete Match | Panitumumab (EGFR mAb inhibitor) | Resistant | COREAD | NCCN guidelines |
| KRAS | G12D), KRAS MUT (G12V | Complete Match | PI3K pathway inhibitor | Resistant | ED | Pre-clinical |
| KRAS | G12D), KRAS MUT (G12V | Complete Match | CDK4/6 inhibitor;MEK inhibitor | Responsive | CESC | Pre-clinical |
| KRAS | G12D), KRAS MUT (G12V | Different Alteration | BRAF inhibitor;MEK inhibitor | Resistant | COREAD | Case report |
| KRAS | G12D), KRAS MUT (G12V | Different Alteration | BRAF inhibitor;EGFR mAb inhibitor | Resistant | COREAD | Case report |
| KRAS | G13R), KRAS MUT (G13A | Complete Match | Panitumumab (EGFR mAb inhibitor) | Resistant | COREAD | FDA guidelines |
| KRAS | G13R), KRAS MUT (G13A | Complete Match | Cetuximab (EGFR mAb inhibitor) | Resistant | COREAD | FDA guidelines |
| KRAS | G13R), KRAS MUT (G13A | Different Mutation | Cetuximab (EGFR mAb inhibitor) | No Responsive | COREAD | Late trials |
| KRAS | G13R), KRAS MUT (G13A | Different Mutation | MEK inhibitor | Responsive | ALL | Pre-clinical |
| KRAS | G13R), KRAS MUT (G13A | Different Mutation | Imatinib (BCR-ABL inhibitor & KIT inhibitor) | Resistant | GIST | Case report |
| KRAS | G13R), KRAS MUT (G13A | Complete Match | MEK inhibitor | Responsive | OV, CER, AML | Pre-clinical |
| KRAS | G13R), KRAS MUT (G13A | Complete Match | Abemaciclib (CDK4/6 inhibitor) | Responsive | L | Early trials |
| KRAS | G13R), KRAS MUT (G13A | Complete Match | MEK inhibitor;PI3K pathway inhibitor | Responsive | COREAD | Pre-clinical |
| KRAS | G13R), KRAS MUT (G13A | Complete Match | Cetuximab (EGFR mAb inhibitor) | Resistant | COREAD | NCCN guidelines |
| KRAS | G13R), KRAS MUT (G13A | Complete Match | Sorafenib;MEK inhibitor (Pan-TK inhibitor;MEK inhibitor) | Responsive | HC | Early trials |
| KRAS | G13R), KRAS MUT (G13A | Complete Match | EGFR inhibitor | Resistant | L | NCCN guidelines |
| KRAS | G13R), KRAS MUT (G13A | Complete Match | CDK4 inhibitor | Responsive | L | Pre-clinical |
| KRAS | G13R), KRAS MUT (G13A | Complete Match | Selumetinib (MEK inhibitor) | Responsive | NSCLC | Early trials |
| KRAS | G13R), KRAS MUT (G13A | Complete Match | BET inhibitor | Responsive | L | Pre-clinical |
| KRAS | G13R), KRAS MUT (G13A | Complete Match | Decitabine;BCL2 inhibitor (Chemotherapy;BCL2 inhibitor) | Responsive | OV | Pre-clinical |
| KRAS | G13R), KRAS MUT (G13A | Complete Match | FAK inhibitor | Responsive | L | Pre-clinical |
| KRAS | G13R), KRAS MUT (G13A | Complete Match | EGFR inhibitor | Resistant | NSCLC | Pre-clinical |
| KRAS | G13R), KRAS MUT (G13A | Complete Match | EZH2 inhibitor | Resistant | CANCER | Pre-clinical |
| KRAS | G13R), KRAS MUT (G13A | Complete Match | MEK inhibitor | Responsive | BT, L | Early trials |
| KRAS | G13R), KRAS MUT (G13A | Complete Match | MEK inhibitor;IGF1R inhibitor | Responsive | COREAD | Pre-clinical |
| KRAS | G13R), KRAS MUT (G13A | Complete Match | MTOR inhibitor;BH3 mimetics | Responsive | COREAD | Pre-clinical |
| KRAS | G13R), KRAS MUT (G13A | Complete Match | EGFR mAb inhibitor;MEK inhibitor | Responsive | COREAD | Pre-clinical |
| KRAS | G13R), KRAS MUT (G13A | Complete Match | pan-RAF inhibitor | Responsive | ED | Case report |
| KRAS | G13R), KRAS MUT (G13A | Complete Match | EGFR mAb inhibitor | Resistant | ST | Pre-clinical |
| KRAS | G13R), KRAS MUT (G13A | Complete Match | PI3K pathway inhibitor;MEK inhibitor | Responsive | L | Early trials |
| KRAS | G13R), KRAS MUT (G13A | Complete Match | Selumetinib (MEK inhibitor) | No Responsive | L | Early trials |
| KRAS | G13R), KRAS MUT (G13A | Complete Match | CDK4/6 inhibitor;MEK inhibitor | Responsive | L | Early trials |
| KRAS | G13R), KRAS MUT (G13A | Complete Match | Trametinib;Ponatinib (MEK inhibitor;BCR-ABL inhibitor & Pan-TK inhibitor) | Responsive | LUAD | Pre-clinical |
| KRAS | G13R), KRAS MUT (G13A | Complete Match | Trastuzumab;Lapatinib (ERBB2 mAb inhibitor;ERBB2 inhibitor) | Resistant | COREAD | Late trials |
| KRAS | G13R), KRAS MUT (G13A | Complete Match | pan-RAF inhibitor | Responsive | L | Early trials |
| KRAS | G13R), KRAS MUT (G13A | Complete Match | JAK/TBK1/IKKε inhibitor | Responsive | L | Pre-clinical |
| KRAS | G13R), KRAS MUT (G13A | Complete Match | FAS inhibitor | Responsive | L | Case report |
| KRAS | G13R), KRAS MUT (G13A | Complete Match | HSP90 inhibitor | Responsive | L | Pre-clinical |
| KRAS | G13R), KRAS MUT (G13A | Complete Match | ERK inhibitor | Responsive | COREAD | Pre-clinical |
| KRAS | G13R), KRAS MUT (G13A | Complete Match | PI3K pathway inhibitor;MEK inhibitor | No Responsive | PA | Early trials |
| KRAS | G13R), KRAS MUT (G13A | Complete Match | MEK inhibitor;BCL-XL inhibitor | Responsive | COREAD | Pre-clinical |
| KRAS | G13R), KRAS MUT (G13A | Complete Match | Gemcitabine;MEK inhibitor (Chemotherapy;MEK inhibitor) | Responsive | PA | Early trials |
| KRAS | G13R), KRAS MUT (G13A | Complete Match | Panitumumab (EGFR mAb inhibitor) | Resistant | COREAD | NCCN guidelines |
| KRAS | G13R), KRAS MUT (G13A | Complete Match | PI3K pathway inhibitor | Resistant | ED | Pre-clinical |
| KRAS | G13R), KRAS MUT (G13A | Complete Match | CDK4/6 inhibitor;MEK inhibitor | Responsive | CESC | Pre-clinical |
| KRAS | G13R), KRAS MUT (G13A | Different Alteration | BRAF inhibitor;MEK inhibitor | Resistant | COREAD | Case report |
| KRAS | G13R), KRAS MUT (G13A | Different Alteration | BRAF inhibitor;EGFR mAb inhibitor | Resistant | COREAD | Case report |
| KRAS | G13D | Complete Match | Panitumumab (EGFR mAb inhibitor) | Resistant | COREAD | FDA guidelines |
| KRAS | G13D | Complete Match | Cetuximab (EGFR mAb inhibitor) | Resistant | COREAD | FDA guidelines |
| KRAS | G13D | Complete Match | Cetuximab (EGFR mAb inhibitor) | No Responsive | COREAD | Late trials |
| KRAS | G13D | Different Mutation | MEK inhibitor | Responsive | ALL | Pre-clinical |
| KRAS | G13D | Different Mutation | Imatinib (BCR-ABL inhibitor & KIT inhibitor) | Resistant | GIST | Case report |
| KRAS | G13D | Complete Match | MEK inhibitor | Responsive | OV, CER, AML | Pre-clinical |
| KRAS | G13D | Complete Match | Abemaciclib (CDK4/6 inhibitor) | Responsive | L | Early trials |
| KRAS | G13D | Complete Match | MEK inhibitor;PI3K pathway inhibitor | Responsive | COREAD | Pre-clinical |
| KRAS | G13D | Complete Match | Cetuximab (EGFR mAb inhibitor) | Resistant | COREAD | NCCN guidelines |
| KRAS | G13D | Complete Match | Sorafenib;MEK inhibitor (Pan-TK inhibitor;MEK inhibitor) | Responsive | HC | Early trials |
| KRAS | G13D | Complete Match | EGFR inhibitor | Resistant | L | NCCN guidelines |
| KRAS | G13D | Complete Match | CDK4 inhibitor | Responsive | L | Pre-clinical |
| KRAS | G13D | Complete Match | Selumetinib (MEK inhibitor) | Responsive | NSCLC | Early trials |
| KRAS | G13D | Complete Match | BET inhibitor | Responsive | L | Pre-clinical |
| KRAS | G13D | Complete Match | Decitabine;BCL2 inhibitor (Chemotherapy;BCL2 inhibitor) | Responsive | OV | Pre-clinical |
| KRAS | G13D | Complete Match | FAK inhibitor | Responsive | L | Pre-clinical |
| KRAS | G13D | Complete Match | EGFR inhibitor | Resistant | NSCLC | Pre-clinical |
| KRAS | G13D | Complete Match | EZH2 inhibitor | Resistant | CANCER | Pre-clinical |
| KRAS | G13D | Complete Match | MEK inhibitor | Responsive | BT, L | Early trials |
| KRAS | G13D | Complete Match | MEK inhibitor;IGF1R inhibitor | Responsive | COREAD | Pre-clinical |
| KRAS | G13D | Complete Match | MTOR inhibitor;BH3 mimetics | Responsive | COREAD | Pre-clinical |
| KRAS | G13D | Complete Match | EGFR mAb inhibitor;MEK inhibitor | Responsive | COREAD | Pre-clinical |
| KRAS | G13D | Complete Match | pan-RAF inhibitor | Responsive | ED | Case report |
| KRAS | G13D | Complete Match | EGFR mAb inhibitor | Resistant | ST | Pre-clinical |
| KRAS | G13D | Complete Match | PI3K pathway inhibitor;MEK inhibitor | Responsive | L | Early trials |
| KRAS | G13D | Complete Match | Selumetinib (MEK inhibitor) | No Responsive | L | Early trials |
| KRAS | G13D | Complete Match | CDK4/6 inhibitor;MEK inhibitor | Responsive | L | Early trials |
| KRAS | G13D | Complete Match | Trametinib;Ponatinib (MEK inhibitor;BCR-ABL inhibitor & Pan-TK inhibitor) | Responsive | LUAD | Pre-clinical |
| KRAS | G13D | Complete Match | Trastuzumab;Lapatinib (ERBB2 mAb inhibitor;ERBB2 inhibitor) | Resistant | COREAD | Late trials |
| KRAS | G13D | Complete Match | pan-RAF inhibitor | Responsive | L | Early trials |
| KRAS | G13D | Complete Match | JAK/TBK1/IKKε inhibitor | Responsive | L | Pre-clinical |
| KRAS | G13D | Complete Match | FAS inhibitor | Responsive | L | Case report |
| KRAS | G13D | Complete Match | HSP90 inhibitor | Responsive | L | Pre-clinical |
| KRAS | G13D | Complete Match | ERK inhibitor | Responsive | COREAD | Pre-clinical |
| KRAS | G13D | Complete Match | PI3K pathway inhibitor;MEK inhibitor | No Responsive | PA | Early trials |
| KRAS | G13D | Complete Match | MEK inhibitor;BCL-XL inhibitor | Responsive | COREAD | Pre-clinical |
| KRAS | G13D | Complete Match | Gemcitabine;MEK inhibitor (Chemotherapy;MEK inhibitor) | Responsive | PA | Early trials |
| KRAS | G13D | Complete Match | Panitumumab (EGFR mAb inhibitor) | Resistant | COREAD | NCCN guidelines |
| KRAS | G13D | Complete Match | PI3K pathway inhibitor | Resistant | ED | Pre-clinical |
| KRAS | G13D | Complete Match | CDK4/6 inhibitor;MEK inhibitor | Responsive | CESC | Pre-clinical |
| KRAS | G13D | Different Alteration | BRAF inhibitor;MEK inhibitor | Resistant | COREAD | Case report |
| KRAS | G13D | Different Alteration | BRAF inhibitor;EGFR mAb inhibitor | Resistant | COREAD | Case report |
| KRAS | G12A | Complete Match | Panitumumab (EGFR mAb inhibitor) | Resistant | COREAD | FDA guidelines |
| KRAS | G12A | Complete Match | Cetuximab (EGFR mAb inhibitor) | Resistant | COREAD | FDA guidelines |
| KRAS | G12A | Different Mutation | Cetuximab (EGFR mAb inhibitor) | No Responsive | COREAD | Late trials |
| KRAS | G12A | Complete Match | MEK inhibitor | Responsive | ALL | Pre-clinical |
| KRAS | G12A | Complete Match | Imatinib (BCR-ABL inhibitor & KIT inhibitor) | Resistant | GIST | Case report |
| KRAS | G12A | Complete Match | MEK inhibitor | Responsive | OV, CER, AML | Pre-clinical |
| KRAS | G12A | Complete Match | Abemaciclib (CDK4/6 inhibitor) | Responsive | L | Early trials |
| KRAS | G12A | Complete Match | MEK inhibitor;PI3K pathway inhibitor | Responsive | COREAD | Pre-clinical |
| KRAS | G12A | Complete Match | Cetuximab (EGFR mAb inhibitor) | Resistant | COREAD | NCCN guidelines |
| KRAS | G12A | Complete Match | Sorafenib;MEK inhibitor (Pan-TK inhibitor;MEK inhibitor) | Responsive | HC | Early trials |
| KRAS | G12A | Complete Match | EGFR inhibitor | Resistant | L | NCCN guidelines |
| KRAS | G12A | Complete Match | CDK4 inhibitor | Responsive | L | Pre-clinical |
| KRAS | G12A | Complete Match | Selumetinib (MEK inhibitor) | Responsive | NSCLC | Early trials |
| KRAS | G12A | Complete Match | BET inhibitor | Responsive | L | Pre-clinical |
| KRAS | G12A | Complete Match | Decitabine;BCL2 inhibitor (Chemotherapy;BCL2 inhibitor) | Responsive | OV | Pre-clinical |
| KRAS | G12A | Complete Match | FAK inhibitor | Responsive | L | Pre-clinical |
| KRAS | G12A | Complete Match | EGFR inhibitor | Resistant | NSCLC | Pre-clinical |
| KRAS | G12A | Complete Match | EZH2 inhibitor | Resistant | CANCER | Pre-clinical |
| KRAS | G12A | Complete Match | MEK inhibitor | Responsive | BT, L | Early trials |
| KRAS | G12A | Complete Match | MEK inhibitor;IGF1R inhibitor | Responsive | COREAD | Pre-clinical |
| KRAS | G12A | Complete Match | MTOR inhibitor;BH3 mimetics | Responsive | COREAD | Pre-clinical |
| KRAS | G12A | Complete Match | EGFR mAb inhibitor;MEK inhibitor | Responsive | COREAD | Pre-clinical |
| KRAS | G12A | Complete Match | pan-RAF inhibitor | Responsive | ED | Case report |
| KRAS | G12A | Complete Match | EGFR mAb inhibitor | Resistant | ST | Pre-clinical |
| KRAS | G12A | Complete Match | PI3K pathway inhibitor;MEK inhibitor | Responsive | L | Early trials |
| KRAS | G12A | Complete Match | Selumetinib (MEK inhibitor) | No Responsive | L | Early trials |
| KRAS | G12A | Complete Match | CDK4/6 inhibitor;MEK inhibitor | Responsive | L | Early trials |
| KRAS | G12A | Complete Match | Trametinib;Ponatinib (MEK inhibitor;BCR-ABL inhibitor & Pan-TK inhibitor) | Responsive | LUAD | Pre-clinical |
| KRAS | G12A | Complete Match | Trastuzumab;Lapatinib (ERBB2 mAb inhibitor;ERBB2 inhibitor) | Resistant | COREAD | Late trials |
| KRAS | G12A | Complete Match | pan-RAF inhibitor | Responsive | L | Early trials |
| KRAS | G12A | Complete Match | JAK/TBK1/IKKε inhibitor | Responsive | L | Pre-clinical |
| KRAS | G12A | Complete Match | FAS inhibitor | Responsive | L | Case report |
| KRAS | G12A | Complete Match | HSP90 inhibitor | Responsive | L | Pre-clinical |
| KRAS | G12A | Complete Match | ERK inhibitor | Responsive | COREAD | Pre-clinical |
| KRAS | G12A | Complete Match | PI3K pathway inhibitor;MEK inhibitor | No Responsive | PA | Early trials |
| KRAS | G12A | Complete Match | MEK inhibitor;BCL-XL inhibitor | Responsive | COREAD | Pre-clinical |
| KRAS | G12A | Complete Match | Gemcitabine;MEK inhibitor (Chemotherapy;MEK inhibitor) | Responsive | PA | Early trials |
| KRAS | G12A | Complete Match | Panitumumab (EGFR mAb inhibitor) | Resistant | COREAD | NCCN guidelines |
| KRAS | G12A | Complete Match | PI3K pathway inhibitor | Resistant | ED | Pre-clinical |
| KRAS | G12A | Complete Match | CDK4/6 inhibitor;MEK inhibitor | Responsive | CESC | Pre-clinical |
| KRAS | G12A | Different Alteration | BRAF inhibitor;MEK inhibitor | Resistant | COREAD | Case report |
| KRAS | G12A | Different Alteration | BRAF inhibitor;EGFR mAb inhibitor | Resistant | COREAD | Case report |
| LRP1B | G649* | Complete Match | Liposomal Doxorubicin (Chemotherapy) | Resistant | OV | Early trials |
| LRP1B | G649* | Different Alteration | Liposomal Doxorubicin (Chemotherapy) | Resistant | OV | Early trials |
| LRP1B | splice_donor_variant | Complete Match | Liposomal Doxorubicin (Chemotherapy) | Resistant | OV | Early trials |
| LRP1B | splice_donor_variant | Different Alteration | Liposomal Doxorubicin (Chemotherapy) | Resistant | OV | Early trials |
| LRP1B | R1632* | Complete Match | Liposomal Doxorubicin (Chemotherapy) | Resistant | OV | Early trials |
| LRP1B | R1632* | Different Alteration | Liposomal Doxorubicin (Chemotherapy) | Resistant | OV | Early trials |
| LRP1B | splice_acceptor_variant | Complete Match | Liposomal Doxorubicin (Chemotherapy) | Resistant | OV | Early trials |
| LRP1B | splice_acceptor_variant | Different Alteration | Liposomal Doxorubicin (Chemotherapy) | Resistant | OV | Early trials |
| MAP2K1 | Q56P | Different Mutation | MEK inhibitor | Resistant | CANCER | Pre-clinical |
| MAP2K1 | Q56P | Different Mutation | MEK inhibitor | Resistant | CM | Case report |
| MAP2K1 | Q56P | Different Mutation | ERK inhibitor | Responsive | CANCER | Pre-clinical |
| MAP2K1 | Q56P | Complete Match | Vemurafenib (BRAF inhibitor) | Resistant | CM | Case report |
| MAP2K1 | Q56P | Complete Match | BRAF inhibitor | Resistant | CM | Case report |
| MAP2K1 | Q56P | Complete Match | Trametinib (MEK inhibitor) | Responsive | CANCER | Pre-clinical |
| MAP2K1 | Q56P | Complete Match | MEK inhibitor | Resistant | CM | Case report |
| MAP2K1 | Q56P | Different Mutation | Panitumumab;Trametinib (EGFR mAb inhibitor;MEK inhibitor) | Responsive | COREAD | Case report |
| MAP2K1 | Q56P | Different Mutation | Selumetinib (MEK inhibitor) | Responsive | OV | Case report |
| MAP2K1 | Q56P | Different Mutation | BRAF inhibitor | Resistant | CM | Early trials |
| MAP2K1 | Q56P | Different Mutation | ERK inhibitor | Responsive | CM | Pre-clinical |
| MAP2K1 | Q56P | Complete Match | EGFR mAb inhibitor | Resistant | COREAD | Case report |
| MAP2K1 | F53L | Different Mutation | MEK inhibitor | Resistant | CANCER | Pre-clinical |
| MAP2K1 | F53L | Different Mutation | novel MEK inhibitor | Responsive | CM | Pre-clinical |
| MAP2K1 | F53L | Different Mutation | MEK inhibitor | Resistant | CM | Case report |
| MAP2K1 | F53L | Different Mutation | ERK inhibitor | Responsive | CANCER | Pre-clinical |
| MAP2K1 | F53L | Different Mutation | Vemurafenib (BRAF inhibitor) | Resistant | CM | Case report |
| MAP2K1 | F53L | Different Mutation | BRAF inhibitor | Resistant | CM | Case report |
| MAP2K1 | F53L | Different Mutation | Trametinib (MEK inhibitor) | Responsive | CANCER | Pre-clinical |
| MAP2K1 | F53L | Different Mutation | Panitumumab;Trametinib (EGFR mAb inhibitor;MEK inhibitor) | Responsive | COREAD | Case report |
| MAP2K1 | F53L | Different Mutation | Selumetinib (MEK inhibitor) | Responsive | OV | Case report |
| MAP2K1 | F53L | Different Mutation | BRAF inhibitor | Resistant | CM | Early trials |
| MAP2K1 | F53L | Different Mutation | ERK inhibitor | Responsive | CM | Pre-clinical |
| MAP2K1 | F53L | Complete Match | EGFR mAb inhibitor | Resistant | COREAD | Case report |
| MET | intron_variant), MET MUT* (intron_variant | Different Alteration | Crizotinib (ALK inhibitor) | Responsive | G | Case report |
| MET | intron_variant | Different Alteration | Crizotinib (ALK inhibitor) | Responsive | G | Case report |
| MET | IntronicBlockSubstitution), MET MUT* (intron_variant | Different Alteration | Crizotinib (ALK inhibitor) | Responsive | G | Case report |
| MET | IntronicBlockSubstitution | Different Alteration | Crizotinib (ALK inhibitor) | Responsive | G | Case report |
| MET | intron_variant), MET MUT* (intron_variant), MET MUT* (intron_variant), MET MUT* (intron_variant), MET MUT* (intron_variant | Different Alteration | Crizotinib (ALK inhibitor) | Responsive | G | Case report |
| MET | intron_variant), MET MUT* (intron_variant), MET MUT* (intron_variant | Different Alteration | Crizotinib (ALK inhibitor) | Responsive | G | Case report |
| MET | T495S | Different Alteration | Crizotinib (ALK inhibitor) | Responsive | G | Case report |
| MET | 3-UTRSNV), MET MUT* (intron_variant | Different Alteration | Crizotinib (ALK inhibitor) | Responsive | G | Case report |
| MET | intron_variant), MET MUT* (intron_variant), MET MUT* (intron_variant), MET MUT* (intron_variant), MET MUT* (intron_variant), MET MUT* (intron_variant), MET MUT* (intron_variant | Different Alteration | Crizotinib (ALK inhibitor) | Responsive | G | Case report |
| MET | S653S | Different Alteration | Crizotinib (ALK inhibitor) | Responsive | G | Case report |
| MET | 3-UTRSNV | Different Alteration | Crizotinib (ALK inhibitor) | Responsive | G | Case report |
| MET | intron_variant), MET MUT* (V910L | Different Alteration | Crizotinib (ALK inhibitor) | Responsive | G | Case report |
| NOTCH1 | intron_variant), NOTCH1 MUT* (intron_variant | Different Alteration | Gamma secretase inhibitor | Responsive | ALL | Pre-clinical |
| NOTCH1 | intron_variant), NOTCH1 MUT* (intron_variant | Different Alteration | Gamma secretase inhibitor | Responsive | BRCA | Pre-clinical |
| NOTCH1 | intron_variant | Different Alteration | Gamma secretase inhibitor | Responsive | ALL | Pre-clinical |
| NOTCH1 | intron_variant | Different Alteration | Gamma secretase inhibitor | Responsive | BRCA | Pre-clinical |
| NOTCH1 | intron_variant), NOTCH1 MUT* (intron_variant), NOTCH1 MUT* (intron_variant | Different Alteration | Gamma secretase inhibitor | Responsive | ALL | Pre-clinical |
| NOTCH1 | intron_variant), NOTCH1 MUT* (intron_variant), NOTCH1 MUT* (intron_variant | Different Alteration | Gamma secretase inhibitor | Responsive | BRCA | Pre-clinical |
| NOTCH1 | C195S | Different Alteration | Gamma secretase inhibitor | Responsive | ALL | Pre-clinical |
| NOTCH1 | C195S | Different Alteration | Gamma secretase inhibitor | Responsive | BRCA | Pre-clinical |
| NOTCH1 | intron_variant), NOTCH1 MUT* (3-UTRSNV | Different Alteration | Gamma secretase inhibitor | Responsive | ALL | Pre-clinical |
| NOTCH1 | intron_variant), NOTCH1 MUT* (3-UTRSNV | Different Alteration | Gamma secretase inhibitor | Responsive | BRCA | Pre-clinical |
| NOTCH1 | intron_variant), NOTCH1 MUT* (A1935A | Different Alteration | Gamma secretase inhibitor | Responsive | ALL | Pre-clinical |
| NOTCH1 | intron_variant), NOTCH1 MUT* (A1935A | Different Alteration | Gamma secretase inhibitor | Responsive | BRCA | Pre-clinical |
| NOTCH1 | D1609D | Different Alteration | Gamma secretase inhibitor | Responsive | ALL | Pre-clinical |
| NOTCH1 | D1609D | Different Alteration | Gamma secretase inhibitor | Responsive | BRCA | Pre-clinical |
| NOTCH2 | intron_variant | Different Alteration | Gamma secretase inhibitor | Responsive | BRCA | Pre-clinical |
| NOTCH2 | intron_variant), NOTCH2 MUT* (intron_variant | Different Alteration | Gamma secretase inhibitor | Responsive | BRCA | Pre-clinical |
| NOTCH2 | T1993T | Different Alteration | Gamma secretase inhibitor | Responsive | BRCA | Pre-clinical |
| NOTCH2 | P1783P | Different Alteration | Gamma secretase inhibitor | Responsive | BRCA | Pre-clinical |
| NOTCH2 | intron_variant), NOTCH2 MUT* (intron_variant), NOTCH2 MUT* (intron_variant | Different Alteration | Gamma secretase inhibitor | Responsive | BRCA | Pre-clinical |
| NOTCH2 | T1163T | Different Alteration | Gamma secretase inhibitor | Responsive | BRCA | Pre-clinical |
| NOTCH2 | R1630C | Different Alteration | Gamma secretase inhibitor | Responsive | BRCA | Pre-clinical |
| NOTCH2 | intron_variant), NOTCH2 MUT* (intron_variant), NOTCH2 MUT* (intron_variant), NOTCH2 MUT* (G2313G | Different Alteration | Gamma secretase inhibitor | Responsive | BRCA | Pre-clinical |
| NOTCH2 | 3-UTRSNV | Different Alteration | Gamma secretase inhibitor | Responsive | BRCA | Pre-clinical |
| NOTCH2 | 5-UTRSNV | Different Alteration | Gamma secretase inhibitor | Responsive | BRCA | Pre-clinical |
| NOTCH2 | intron_variant), NOTCH2 MUT* (intron_variant), NOTCH2 MUT* (intron_variant), NOTCH2 MUT* (intron_variant), NOTCH2 MUT* (intron_variant | Different Alteration | Gamma secretase inhibitor | Responsive | BRCA | Pre-clinical |
| NOTCH2 | Q868P | Different Alteration | Gamma secretase inhibitor | Responsive | BRCA | Pre-clinical |
| NOTCH2 | intron_variant), NOTCH2 MUT* (intron_variant), NOTCH2 MUT* (A1154V | Different Alteration | Gamma secretase inhibitor | Responsive | BRCA | Pre-clinical |
| NRAS | Q61R | Complete Match | Cetuximab (EGFR mAb inhibitor) | Resistant | COREAD | NCCN guidelines |
| NRAS | Q61R | Complete Match | Panitumumab (EGFR mAb inhibitor) | Resistant | COREAD | FDA guidelines |
| NRAS | Q61R | Complete Match | MEK inhibitor | Responsive | CM | Late trials |
| NRAS | Q61R | Complete Match | BRAF inhibitor | Resistant | CM | Early trials |
| NRAS | Q61R | Complete Match | CDK4/6 inhibitor;MEK inhibitor | Responsive | CM | Early trials |
| NRAS | Q61R | Complete Match | HSP90 inhibitor | Responsive | CM | Pre-clinical |
| NRAS | Q61R | Complete Match | MEK inhibitor | Responsive | AML, LUAD, ALL | Pre-clinical |
| NRAS | Q61R | Complete Match | Pan-RAF inhibitor | Responsive | CM | Case report |
| NRAS | Q61R | Complete Match | Sorafenib;MEK inhibitor (Pan-TK inhibitor;MEK inhibitor) | Responsive | HC | Early trials |
| NRAS | Q61R | Complete Match | ERK inhibitor | Responsive | CM | Case report |
| NRAS | Q61R | Complete Match | MEK inhibitor +/- PI3K pathway inhibitor | Responsive | COREAD | Pre-clinical |
| NRAS | Q61R | Complete Match | Vemurafenib (BRAF inhibitor) | Resistant | CM | Pre-clinical |
| NRAS | Q61R | Complete Match | PI3K pathway inhibitor;MEK inhibitor | Responsive | MYMA | Pre-clinical |
| NRAS | Q61R | Different Mutation | ERK inhibitor | Responsive | CANCER | Pre-clinical |
| NRAS | Y64_Q70delinsVSHE | Different Mutation | Cetuximab (EGFR mAb inhibitor) | Resistant | COREAD | NCCN guidelines |
| NRAS | Y64_Q70delinsVSHE | Different Mutation | Panitumumab (EGFR mAb inhibitor) | Resistant | COREAD | FDA guidelines |
| NRAS | Y64_Q70delinsVSHE | Different Mutation | MEK inhibitor | Responsive | CM | Late trials |
| NRAS | Y64_Q70delinsVSHE | Different Mutation | BRAF inhibitor | Resistant | CM | Early trials |
| NRAS | Y64_Q70delinsVSHE | Complete Match | CDK4/6 inhibitor;MEK inhibitor | Responsive | CM | Early trials |
| NRAS | Y64_Q70delinsVSHE | Complete Match | HSP90 inhibitor | Responsive | CM | Pre-clinical |
| NRAS | Y64_Q70delinsVSHE | Complete Match | MEK inhibitor | Responsive | AML, LUAD, ALL | Pre-clinical |
| NRAS | Y64_Q70delinsVSHE | Complete Match | Pan-RAF inhibitor | Responsive | CM | Case report |
| NRAS | Y64_Q70delinsVSHE | Complete Match | Sorafenib;MEK inhibitor (Pan-TK inhibitor;MEK inhibitor) | Responsive | HC | Early trials |
| NRAS | Y64_Q70delinsVSHE | Complete Match | ERK inhibitor | Responsive | CM | Case report |
| NRAS | Y64_Q70delinsVSHE | Complete Match | MEK inhibitor +/- PI3K pathway inhibitor | Responsive | COREAD | Pre-clinical |
| NRAS | Y64_Q70delinsVSHE | Complete Match | Vemurafenib (BRAF inhibitor) | Resistant | CM | Pre-clinical |
| NRAS | Y64_Q70delinsVSHE | Complete Match | PI3K pathway inhibitor;MEK inhibitor | Responsive | MYMA | Pre-clinical |
| NRAS | Y64_Q70delinsVSHE | Different Mutation | ERK inhibitor | Responsive | CANCER | Pre-clinical |
| NRG1 | intron_variant | Different Alteration | Lapatinib (ERBB2 inhibitor) | Responsive | LUAD | Pre-clinical |
| NRG1 | intron_variant | Different Alteration | Afatinib (ERBB2 & EGFR inhibitor) | Responsive | LUAD | Case report |
| NRG1 | intron_variant), NRG1 MUT* (IntronicBlockSubstitution), NRG1 MUT* (intron_variant | Different Alteration | Lapatinib (ERBB2 inhibitor) | Responsive | LUAD | Pre-clinical |
| NRG1 | intron_variant), NRG1 MUT* (IntronicBlockSubstitution), NRG1 MUT* (intron_variant | Different Alteration | Afatinib (ERBB2 & EGFR inhibitor) | Responsive | LUAD | Case report |
| NRG1 | intron_variant), NRG1 MUT* (intron_variant | Different Alteration | Lapatinib (ERBB2 inhibitor) | Responsive | LUAD | Pre-clinical |
| NRG1 | intron_variant), NRG1 MUT* (intron_variant | Different Alteration | Afatinib (ERBB2 & EGFR inhibitor) | Responsive | LUAD | Case report |
| NRG1 | intron_variant), NRG1 MUT* (intron_variant), NRG1 MUT* (intron_variant), NRG1 MUT* (intron_variant), NRG1 MUT* (intron_variant | Different Alteration | Lapatinib (ERBB2 inhibitor) | Responsive | LUAD | Pre-clinical |
| NRG1 | intron_variant), NRG1 MUT* (intron_variant), NRG1 MUT* (intron_variant), NRG1 MUT* (intron_variant), NRG1 MUT* (intron_variant | Different Alteration | Afatinib (ERBB2 & EGFR inhibitor) | Responsive | LUAD | Case report |
| NRG1 | intron_variant), NRG1 MUT* (intron_variant), NRG1 MUT* (intron_variant), NRG1 MUT* (intron_variant | Different Alteration | Lapatinib (ERBB2 inhibitor) | Responsive | LUAD | Pre-clinical |
| NRG1 | intron_variant), NRG1 MUT* (intron_variant), NRG1 MUT* (intron_variant), NRG1 MUT* (intron_variant | Different Alteration | Afatinib (ERBB2 & EGFR inhibitor) | Responsive | LUAD | Case report |
| NRG1 | intron_variant), NRG1 MUT* (intron_variant), NRG1 MUT* (intron_variant | Different Alteration | Lapatinib (ERBB2 inhibitor) | Responsive | LUAD | Pre-clinical |
| NRG1 | intron_variant), NRG1 MUT* (intron_variant), NRG1 MUT* (intron_variant | Different Alteration | Afatinib (ERBB2 & EGFR inhibitor) | Responsive | LUAD | Case report |
| NRG1 | intron_variant), NRG1 MUT* (intron_variant), NRG1 MUT* (IntronicBlockSubstitution), NRG1 MUT* (IntronicBlockSubstitution | Different Alteration | Lapatinib (ERBB2 inhibitor) | Responsive | LUAD | Pre-clinical |
| NRG1 | intron_variant), NRG1 MUT* (intron_variant), NRG1 MUT* (IntronicBlockSubstitution), NRG1 MUT* (IntronicBlockSubstitution | Different Alteration | Afatinib (ERBB2 & EGFR inhibitor) | Responsive | LUAD | Case report |
| NRG1 | intron_variant), NRG1 MUT* (intron_variant), NRG1 MUT* (intron_variant), NRG1 MUT* (intron_variant), NRG1 MUT* (intron_variant), NRG1 MUT* (intron_variant), NRG1 MUT* (intron_variant | Different Alteration | Lapatinib (ERBB2 inhibitor) | Responsive | LUAD | Pre-clinical |
| NRG1 | intron_variant), NRG1 MUT* (intron_variant), NRG1 MUT* (intron_variant), NRG1 MUT* (intron_variant), NRG1 MUT* (intron_variant), NRG1 MUT* (intron_variant), NRG1 MUT* (intron_variant | Different Alteration | Afatinib (ERBB2 & EGFR inhibitor) | Responsive | LUAD | Case report |
| NRG1 | L522F | Different Alteration | Lapatinib (ERBB2 inhibitor) | Responsive | LUAD | Pre-clinical |
| NRG1 | L522F | Different Alteration | Afatinib (ERBB2 & EGFR inhibitor) | Responsive | LUAD | Case report |
| NRG1 | intron_variant), NRG1 MUT* (intron_variant), NRG1 MUT* (intron_variant), NRG1 MUT* (intron_variant), NRG1 MUT* (intron_variant), NRG1 MUT* (intron_variant), NRG1 MUT* (intron_variant), NRG1 MUT* (intron_variant), NRG1 MUT* (intron_variant), NRG1 MUT* | Different Alteration | Lapatinib (ERBB2 inhibitor) | Responsive | LUAD | Pre-clinical |
| NRG1 | intron_variant), NRG1 MUT* (intron_variant), NRG1 MUT* (intron_variant), NRG1 MUT* (intron_variant), NRG1 MUT* (intron_variant), NRG1 MUT* (intron_variant), NRG1 MUT* (intron_variant), NRG1 MUT* (intron_variant), NRG1 MUT* (intron_variant), NRG1 MUT* | Different Alteration | Afatinib (ERBB2 & EGFR inhibitor) | Responsive | LUAD | Case report |
| NRG1 | P482T), NRG1 MUT* (V261V | Different Alteration | Lapatinib (ERBB2 inhibitor) | Responsive | LUAD | Pre-clinical |
| NRG1 | P482T), NRG1 MUT* (V261V | Different Alteration | Afatinib (ERBB2 & EGFR inhibitor) | Responsive | LUAD | Case report |
| NRG1 | A511V | Different Alteration | Lapatinib (ERBB2 inhibitor) | Responsive | LUAD | Pre-clinical |
| NRG1 | A511V | Different Alteration | Afatinib (ERBB2 & EGFR inhibitor) | Responsive | LUAD | Case report |
| NRG1 | intron_variant), NRG1 MUT* (intron_variant), NRG1 MUT* (intron_variant), NRG1 MUT* (intron_variant), NRG1 MUT* (intron_variant), NRG1 MUT* (intron_variant | Different Alteration | Lapatinib (ERBB2 inhibitor) | Responsive | LUAD | Pre-clinical |
| NRG1 | intron_variant), NRG1 MUT* (intron_variant), NRG1 MUT* (intron_variant), NRG1 MUT* (intron_variant), NRG1 MUT* (intron_variant), NRG1 MUT* (intron_variant | Different Alteration | Afatinib (ERBB2 & EGFR inhibitor) | Responsive | LUAD | Case report |
| NRG1 | intron_variant), NRG1 MUT* (intron_variant), NRG1 MUT* (5-UTRSNV | Different Alteration | Lapatinib (ERBB2 inhibitor) | Responsive | LUAD | Pre-clinical |
| NRG1 | intron_variant), NRG1 MUT* (intron_variant), NRG1 MUT* (5-UTRSNV | Different Alteration | Afatinib (ERBB2 & EGFR inhibitor) | Responsive | LUAD | Case report |
| NRG1 | IntronicBlockSubstitution | Different Alteration | Lapatinib (ERBB2 inhibitor) | Responsive | LUAD | Pre-clinical |
| NRG1 | IntronicBlockSubstitution | Different Alteration | Afatinib (ERBB2 & EGFR inhibitor) | Responsive | LUAD | Case report |
| NTRK1 | intron_variant | Different Alteration | Crizotinib (ALK inhibitor) | Responsive | LUAD | Case report |
| NTRK1 | intron_variant | Different Alteration | Pan-TKR inhibitor | Responsive | CANCER | Early trials |
| NTRK1 | intron_variant | Different Alteration | Pan-TK inhibitor | Responsive | LUAD | Pre-clinical |
| NTRK1 | intron_variant | Different Alteration | Pan-TK inhibitor | Responsive | COREAD | Case report |
| NTRK1 | E324* | Different Alteration | Crizotinib (ALK inhibitor) | Responsive | LUAD | Case report |
| NTRK1 | E324* | Different Alteration | Pan-TKR inhibitor | Responsive | CANCER | Early trials |
| NTRK1 | E324* | Different Alteration | Pan-TK inhibitor | Responsive | LUAD | Pre-clinical |
| NTRK1 | E324* | Different Alteration | Pan-TK inhibitor | Responsive | COREAD | Case report |
| NTRK1 | L731L), NTRK1 MUT* (L700L), NTRK1 MUT* (T687T), NTRK1 MUT* (intron_variant), NTRK1 MUT* (intron_variant | Different Alteration | Crizotinib (ALK inhibitor) | Responsive | LUAD | Case report |
| NTRK1 | L731L), NTRK1 MUT* (L700L), NTRK1 MUT* (T687T), NTRK1 MUT* (intron_variant), NTRK1 MUT* (intron_variant | Different Alteration | Pan-TKR inhibitor | Responsive | CANCER | Early trials |
| NTRK1 | L731L), NTRK1 MUT* (L700L), NTRK1 MUT* (T687T), NTRK1 MUT* (intron_variant), NTRK1 MUT* (intron_variant | Different Alteration | Pan-TK inhibitor | Responsive | LUAD | Pre-clinical |
| NTRK1 | L731L), NTRK1 MUT* (L700L), NTRK1 MUT* (T687T), NTRK1 MUT* (intron_variant), NTRK1 MUT* (intron_variant | Different Alteration | Pan-TK inhibitor | Responsive | COREAD | Case report |
| NTRK1 | R559C | Different Alteration | Crizotinib (ALK inhibitor) | Responsive | LUAD | Case report |
| NTRK1 | R559C | Different Alteration | Pan-TKR inhibitor | Responsive | CANCER | Early trials |
| NTRK1 | R559C | Different Alteration | Pan-TK inhibitor | Responsive | LUAD | Pre-clinical |
| NTRK1 | R559C | Different Alteration | Pan-TK inhibitor | Responsive | COREAD | Case report |
| NTRK1 | intron_variant), NTRK1 MUT* (intron_variant), NTRK1 MUT* (intron_variant | Different Alteration | Crizotinib (ALK inhibitor) | Responsive | LUAD | Case report |
| NTRK1 | intron_variant), NTRK1 MUT* (intron_variant), NTRK1 MUT* (intron_variant | Different Alteration | Pan-TKR inhibitor | Responsive | CANCER | Early trials |
| NTRK1 | intron_variant), NTRK1 MUT* (intron_variant), NTRK1 MUT* (intron_variant | Different Alteration | Pan-TK inhibitor | Responsive | LUAD | Pre-clinical |
| NTRK1 | intron_variant), NTRK1 MUT* (intron_variant), NTRK1 MUT* (intron_variant | Different Alteration | Pan-TK inhibitor | Responsive | COREAD | Case report |
| NTRK1 | R347H | Different Alteration | Crizotinib (ALK inhibitor) | Responsive | LUAD | Case report |
| NTRK1 | R347H | Different Alteration | Pan-TKR inhibitor | Responsive | CANCER | Early trials |
| NTRK1 | R347H | Different Alteration | Pan-TK inhibitor | Responsive | LUAD | Pre-clinical |
| NTRK1 | R347H | Different Alteration | Pan-TK inhibitor | Responsive | COREAD | Case report |
| NTRK1 | Q765Q | Different Alteration | Crizotinib (ALK inhibitor) | Responsive | LUAD | Case report |
| NTRK1 | Q765Q | Different Alteration | Pan-TKR inhibitor | Responsive | CANCER | Early trials |
| NTRK1 | Q765Q | Different Alteration | Pan-TK inhibitor | Responsive | LUAD | Pre-clinical |
| NTRK1 | Q765Q | Different Alteration | Pan-TK inhibitor | Responsive | COREAD | Case report |
| NTRK1 | V710M | Different Alteration | Crizotinib (ALK inhibitor) | Responsive | LUAD | Case report |
| NTRK1 | V710M | Different Alteration | Pan-TKR inhibitor | Responsive | CANCER | Early trials |
| NTRK1 | V710M | Different Alteration | Pan-TK inhibitor | Responsive | LUAD | Pre-clinical |
| NTRK1 | V710M | Different Alteration | Pan-TK inhibitor | Responsive | COREAD | Case report |
| NTRK1 | P335L), NTRK1 MUT* (intron_variant | Different Alteration | Crizotinib (ALK inhibitor) | Responsive | LUAD | Case report |
| NTRK1 | P335L), NTRK1 MUT* (intron_variant | Different Alteration | Pan-TKR inhibitor | Responsive | CANCER | Early trials |
| NTRK1 | P335L), NTRK1 MUT* (intron_variant | Different Alteration | Pan-TK inhibitor | Responsive | LUAD | Pre-clinical |
| NTRK1 | P335L), NTRK1 MUT* (intron_variant | Different Alteration | Pan-TK inhibitor | Responsive | COREAD | Case report |
| NTRK3 | intron_variant | Different Alteration | IGF1R inhibitor | Responsive | BRCA | Pre-clinical |
| NTRK3 | intron_variant | Different Alteration | PI3K pathway inhibitor | Responsive | BRCA | Pre-clinical |
| NTRK3 | intron_variant | Different Alteration | Midostaurin (Pan-TK inhibitor) | Responsive | BRCA | Pre-clinical |
| NTRK3 | intron_variant), NTRK3 MUT* (intron_variant), NTRK3 MUT* (intron_variant), NTRK3 MUT* (intron_variant), NTRK3 MUT* (intron_variant), NTRK3 MUT* (intron_variant | Different Alteration | IGF1R inhibitor | Responsive | BRCA | Pre-clinical |
| NTRK3 | intron_variant), NTRK3 MUT* (intron_variant), NTRK3 MUT* (intron_variant), NTRK3 MUT* (intron_variant), NTRK3 MUT* (intron_variant), NTRK3 MUT* (intron_variant | Different Alteration | PI3K pathway inhibitor | Responsive | BRCA | Pre-clinical |
| NTRK3 | intron_variant), NTRK3 MUT* (intron_variant), NTRK3 MUT* (intron_variant), NTRK3 MUT* (intron_variant), NTRK3 MUT* (intron_variant), NTRK3 MUT* (intron_variant | Different Alteration | Midostaurin (Pan-TK inhibitor) | Responsive | BRCA | Pre-clinical |
| NTRK3 | intron_variant), NTRK3 MUT* (intron_variant | Different Alteration | IGF1R inhibitor | Responsive | BRCA | Pre-clinical |
| NTRK3 | intron_variant), NTRK3 MUT* (intron_variant | Different Alteration | PI3K pathway inhibitor | Responsive | BRCA | Pre-clinical |
| NTRK3 | intron_variant), NTRK3 MUT* (intron_variant | Different Alteration | Midostaurin (Pan-TK inhibitor) | Responsive | BRCA | Pre-clinical |
| NTRK3 | intron_variant), NTRK3 MUT* (intron_variant), NTRK3 MUT* (intron_variant), NTRK3 MUT* (intron_variant), NTRK3 MUT* (intron_variant | Different Alteration | IGF1R inhibitor | Responsive | BRCA | Pre-clinical |
| NTRK3 | intron_variant), NTRK3 MUT* (intron_variant), NTRK3 MUT* (intron_variant), NTRK3 MUT* (intron_variant), NTRK3 MUT* (intron_variant | Different Alteration | PI3K pathway inhibitor | Responsive | BRCA | Pre-clinical |
| NTRK3 | intron_variant), NTRK3 MUT* (intron_variant), NTRK3 MUT* (intron_variant), NTRK3 MUT* (intron_variant), NTRK3 MUT* (intron_variant | Different Alteration | Midostaurin (Pan-TK inhibitor) | Responsive | BRCA | Pre-clinical |
| NTRK3 | intron_variant), NTRK3 MUT* (intron_variant), NTRK3 MUT* (intron_variant), NTRK3 MUT* (intron_variant), NTRK3 MUT* (intron_variant), NTRK3 MUT* (intron_variant), NTRK3 MUT* (intron_variant | Different Alteration | IGF1R inhibitor | Responsive | BRCA | Pre-clinical |
| NTRK3 | intron_variant), NTRK3 MUT* (intron_variant), NTRK3 MUT* (intron_variant), NTRK3 MUT* (intron_variant), NTRK3 MUT* (intron_variant), NTRK3 MUT* (intron_variant), NTRK3 MUT* (intron_variant | Different Alteration | PI3K pathway inhibitor | Responsive | BRCA | Pre-clinical |
| NTRK3 | intron_variant), NTRK3 MUT* (intron_variant), NTRK3 MUT* (intron_variant), NTRK3 MUT* (intron_variant), NTRK3 MUT* (intron_variant), NTRK3 MUT* (intron_variant), NTRK3 MUT* (intron_variant | Different Alteration | Midostaurin (Pan-TK inhibitor) | Responsive | BRCA | Pre-clinical |
| NTRK3 | intron_variant), NTRK3 MUT* (intron_variant), NTRK3 MUT* (intron_variant), NTRK3 MUT* (intron_variant | Different Alteration | IGF1R inhibitor | Responsive | BRCA | Pre-clinical |
| NTRK3 | intron_variant), NTRK3 MUT* (intron_variant), NTRK3 MUT* (intron_variant), NTRK3 MUT* (intron_variant | Different Alteration | PI3K pathway inhibitor | Responsive | BRCA | Pre-clinical |
| NTRK3 | intron_variant), NTRK3 MUT* (intron_variant), NTRK3 MUT* (intron_variant), NTRK3 MUT* (intron_variant | Different Alteration | Midostaurin (Pan-TK inhibitor) | Responsive | BRCA | Pre-clinical |
| NTRK3 | intron_variant), NTRK3 MUT* (intron_variant), NTRK3 MUT* (intron_variant | Different Alteration | IGF1R inhibitor | Responsive | BRCA | Pre-clinical |
| NTRK3 | intron_variant), NTRK3 MUT* (intron_variant), NTRK3 MUT* (intron_variant | Different Alteration | PI3K pathway inhibitor | Responsive | BRCA | Pre-clinical |
| NTRK3 | intron_variant), NTRK3 MUT* (intron_variant), NTRK3 MUT* (intron_variant | Different Alteration | Midostaurin (Pan-TK inhibitor) | Responsive | BRCA | Pre-clinical |
| NTRK3 | intron_variant), NTRK3 MUT* (intron_variant), NTRK3 MUT* (intron_variant), NTRK3 MUT* (intron_variant), NTRK3 MUT* (intron_variant), NTRK3 MUT* (intron_variant), NTRK3 MUT* (intron_variant), NTRK3 MUT* (intron_variant), NTRK3 MUT* (intron_variant | Different Alteration | IGF1R inhibitor | Responsive | BRCA | Pre-clinical |
| NTRK3 | intron_variant), NTRK3 MUT* (intron_variant), NTRK3 MUT* (intron_variant), NTRK3 MUT* (intron_variant), NTRK3 MUT* (intron_variant), NTRK3 MUT* (intron_variant), NTRK3 MUT* (intron_variant), NTRK3 MUT* (intron_variant), NTRK3 MUT* (intron_variant | Different Alteration | PI3K pathway inhibitor | Responsive | BRCA | Pre-clinical |
| NTRK3 | intron_variant), NTRK3 MUT* (intron_variant), NTRK3 MUT* (intron_variant), NTRK3 MUT* (intron_variant), NTRK3 MUT* (intron_variant), NTRK3 MUT* (intron_variant), NTRK3 MUT* (intron_variant), NTRK3 MUT* (intron_variant), NTRK3 MUT* (intron_variant | Different Alteration | Midostaurin (Pan-TK inhibitor) | Responsive | BRCA | Pre-clinical |
| NTRK3 | intron_variant), NTRK3 MUT* (intron_variant), NTRK3 MUT* (intron_variant), NTRK3 MUT* (intron_variant), NTRK3 MUT* (intron_variant), NTRK3 MUT* (intron_variant), NTRK3 MUT* (intron_variant), NTRK3 MUT* (intron_variant | Different Alteration | IGF1R inhibitor | Responsive | BRCA | Pre-clinical |
| NTRK3 | intron_variant), NTRK3 MUT* (intron_variant), NTRK3 MUT* (intron_variant), NTRK3 MUT* (intron_variant), NTRK3 MUT* (intron_variant), NTRK3 MUT* (intron_variant), NTRK3 MUT* (intron_variant), NTRK3 MUT* (intron_variant | Different Alteration | PI3K pathway inhibitor | Responsive | BRCA | Pre-clinical |
| NTRK3 | intron_variant), NTRK3 MUT* (intron_variant), NTRK3 MUT* (intron_variant), NTRK3 MUT* (intron_variant), NTRK3 MUT* (intron_variant), NTRK3 MUT* (intron_variant), NTRK3 MUT* (intron_variant), NTRK3 MUT* (intron_variant | Different Alteration | Midostaurin (Pan-TK inhibitor) | Responsive | BRCA | Pre-clinical |
| NTRK3 | intron_variant), NTRK3 MUT* (intron_variant), NTRK3 MUT* (intron_variant), NTRK3 MUT* (P467P), NTRK3 MUT* (intron_variant), NTRK3 MUT* (intron_variant), NTRK3 MUT* (intron_variant | Different Alteration | IGF1R inhibitor | Responsive | BRCA | Pre-clinical |
| NTRK3 | intron_variant), NTRK3 MUT* (intron_variant), NTRK3 MUT* (intron_variant), NTRK3 MUT* (P467P), NTRK3 MUT* (intron_variant), NTRK3 MUT* (intron_variant), NTRK3 MUT* (intron_variant | Different Alteration | PI3K pathway inhibitor | Responsive | BRCA | Pre-clinical |
| NTRK3 | intron_variant), NTRK3 MUT* (intron_variant), NTRK3 MUT* (intron_variant), NTRK3 MUT* (P467P), NTRK3 MUT* (intron_variant), NTRK3 MUT* (intron_variant), NTRK3 MUT* (intron_variant | Different Alteration | Midostaurin (Pan-TK inhibitor) | Responsive | BRCA | Pre-clinical |
| NTRK3 | intron_variant), NTRK3 MUT* (intron_variant), NTRK3 MUT* (intron_variant), NTRK3 MUT* (intron_variant), NTRK3 MUT* (intron_variant), NTRK3 MUT* (intron_variant), NTRK3 MUT* (intron_variant), NTRK3 MUT* (intron_variant), NTRK3 MUT* (intron_variant), N | Different Alteration | IGF1R inhibitor | Responsive | BRCA | Pre-clinical |
| NTRK3 | intron_variant), NTRK3 MUT* (intron_variant), NTRK3 MUT* (intron_variant), NTRK3 MUT* (intron_variant), NTRK3 MUT* (intron_variant), NTRK3 MUT* (intron_variant), NTRK3 MUT* (intron_variant), NTRK3 MUT* (intron_variant), NTRK3 MUT* (intron_variant), N | Different Alteration | PI3K pathway inhibitor | Responsive | BRCA | Pre-clinical |
| NTRK3 | intron_variant), NTRK3 MUT* (intron_variant), NTRK3 MUT* (intron_variant), NTRK3 MUT* (intron_variant), NTRK3 MUT* (intron_variant), NTRK3 MUT* (intron_variant), NTRK3 MUT* (intron_variant), NTRK3 MUT* (intron_variant), NTRK3 MUT* (intron_variant), N | Different Alteration | Midostaurin (Pan-TK inhibitor) | Responsive | BRCA | Pre-clinical |
| NTRK3 | intron_variant), NTRK3 MUT* (T149T), NTRK3 MUT* (intron_variant), NTRK3 MUT* (intron_variant), NTRK3 MUT* (intron_variant), NTRK3 MUT* (intron_variant), NTRK3 MUT* (intron_variant), NTRK3 MUT* (intron_variant | Different Alteration | IGF1R inhibitor | Responsive | BRCA | Pre-clinical |
| NTRK3 | intron_variant), NTRK3 MUT* (T149T), NTRK3 MUT* (intron_variant), NTRK3 MUT* (intron_variant), NTRK3 MUT* (intron_variant), NTRK3 MUT* (intron_variant), NTRK3 MUT* (intron_variant), NTRK3 MUT* (intron_variant | Different Alteration | PI3K pathway inhibitor | Responsive | BRCA | Pre-clinical |
| NTRK3 | intron_variant), NTRK3 MUT* (T149T), NTRK3 MUT* (intron_variant), NTRK3 MUT* (intron_variant), NTRK3 MUT* (intron_variant), NTRK3 MUT* (intron_variant), NTRK3 MUT* (intron_variant), NTRK3 MUT* (intron_variant | Different Alteration | Midostaurin (Pan-TK inhibitor) | Responsive | BRCA | Pre-clinical |
| NTRK3 | intron_variant), NTRK3 MUT* (intron_variant), NTRK3 MUT* (intron_variant), NTRK3 MUT* (intron_variant), NTRK3 MUT* (intron_variant), NTRK3 MUT* (C607* | Different Alteration | IGF1R inhibitor | Responsive | BRCA | Pre-clinical |
| NTRK3 | intron_variant), NTRK3 MUT* (intron_variant), NTRK3 MUT* (intron_variant), NTRK3 MUT* (intron_variant), NTRK3 MUT* (intron_variant), NTRK3 MUT* (C607* | Different Alteration | PI3K pathway inhibitor | Responsive | BRCA | Pre-clinical |
| NTRK3 | intron_variant), NTRK3 MUT* (intron_variant), NTRK3 MUT* (intron_variant), NTRK3 MUT* (intron_variant), NTRK3 MUT* (intron_variant), NTRK3 MUT* (C607* | Different Alteration | Midostaurin (Pan-TK inhibitor) | Responsive | BRCA | Pre-clinical |
| NTRK3 | intron_variant), NTRK3 MUT* (intron_variant), NTRK3 MUT* (intron_variant), NTRK3 MUT* (3-UTRSNV | Different Alteration | IGF1R inhibitor | Responsive | BRCA | Pre-clinical |
| NTRK3 | intron_variant), NTRK3 MUT* (intron_variant), NTRK3 MUT* (intron_variant), NTRK3 MUT* (3-UTRSNV | Different Alteration | PI3K pathway inhibitor | Responsive | BRCA | Pre-clinical |
| NTRK3 | intron_variant), NTRK3 MUT* (intron_variant), NTRK3 MUT* (intron_variant), NTRK3 MUT* (3-UTRSNV | Different Alteration | Midostaurin (Pan-TK inhibitor) | Responsive | BRCA | Pre-clinical |
| NTRK3 | intron_variant), NTRK3 MUT* (F517L), NTRK3 MUT* (intron_variant), NTRK3 MUT* (intron_variant | Different Alteration | IGF1R inhibitor | Responsive | BRCA | Pre-clinical |
| NTRK3 | intron_variant), NTRK3 MUT* (F517L), NTRK3 MUT* (intron_variant), NTRK3 MUT* (intron_variant | Different Alteration | PI3K pathway inhibitor | Responsive | BRCA | Pre-clinical |
| NTRK3 | intron_variant), NTRK3 MUT* (F517L), NTRK3 MUT* (intron_variant), NTRK3 MUT* (intron_variant | Different Alteration | Midostaurin (Pan-TK inhibitor) | Responsive | BRCA | Pre-clinical |
| PBRM1 | Q64* | Different Alteration | Everolimus (MTOR inhibitor) | Responsive | R | Pre-clinical |
| PBRM1 | Q64* | Complete Match | anti-PD1 inhibitor | Responsive | RCCC | Early trials |
| PBRM1 | Q64* | Complete Match | Everolimus (MTOR inhibitor) | Responsive | RCC | Early trials |
| PBRM1 | Q64* | Complete Match | EZH2 inhibitor | Responsive | CANCER | Pre-clinical |
| PBRM1 | Q64* | Different Alteration | EZH2 inhibitor | Responsive | CANCER | Pre-clinical |
| PDGFB | 3-UTRSNV | Different Alteration | Imatinib (BCR-ABL inhibitor & KIT inhibitor) | Responsive | DFS | FDA guidelines |
| PDGFB | intron_variant | Different Alteration | Imatinib (BCR-ABL inhibitor & KIT inhibitor) | Responsive | DFS | FDA guidelines |
| PDGFRA | intron_variant), PDGFRA MUT* (IntronicBlockSubstitution), PDGFRA MUT* (intron_variant | Different Alteration | Imatinib (BCR-ABL inhibitor & KIT inhibitor) | Responsive | MDPS, MDS, ECL, HES | FDA guidelines |
| PDGFRA | intron_variant | Different Alteration | Imatinib (BCR-ABL inhibitor & KIT inhibitor) | Responsive | MDPS, MDS, ECL, HES | FDA guidelines |
| PDGFRA | intron_variant), PDGFRA MUT* (intron_variant), PDGFRA MUT* (intron_variant | Different Alteration | Imatinib (BCR-ABL inhibitor & KIT inhibitor) | Responsive | MDPS, MDS, ECL, HES | FDA guidelines |
| PDGFRA | intron_variant), PDGFRA MUT* (intron_variant | Different Alteration | Imatinib (BCR-ABL inhibitor & KIT inhibitor) | Responsive | MDPS, MDS, ECL, HES | FDA guidelines |
| PDGFRA | N995N | Different Alteration | Imatinib (BCR-ABL inhibitor & KIT inhibitor) | Responsive | MDPS, MDS, ECL, HES | FDA guidelines |
| PDGFRA | 3-UTRSNV), PDGFRA MUT* (intron_variant | Different Alteration | Imatinib (BCR-ABL inhibitor & KIT inhibitor) | Responsive | MDPS, MDS, ECL, HES | FDA guidelines |
| PDGFRA | intron_variant), PDGFRA MUT* (IntronicBlockSubstitution | Different Alteration | Imatinib (BCR-ABL inhibitor & KIT inhibitor) | Responsive | MDPS, MDS, ECL, HES | FDA guidelines |
| PIK3CA | R93W | Different Mutation | BRAF inhibitor | Resistant | CM | Case report |
| PIK3CA | R93W | Complete Match | PI3K pathway inhibitor | Responsive | BLCA, HNC | Case report |
| PIK3CA | R93W | Complete Match | PI3K pathway inhibitor | Responsive | HNSC | Case report |
| PIK3CA | R93W | Complete Match | PI3K pathway inhibitor | Responsive | G, THCA | Pre-clinical |
| PIK3CA | R93W | Complete Match | Everolimus;Trastuzumab;Chemotherapy (MTOR inhibitor;ERBB2 mAb inhibitor;Chemotherapy) | Responsive | BRCA | Late trials |
| PIK3CA | R93W | Complete Match | PI3K pathway inhibitor | Responsive | COREAD | Pre-clinical |
| PIK3CA | R93W | Complete Match | PI3K pathway inhibitor | Responsive | ED, OV, CESC | Early trials |
| PIK3CA | R93W | Complete Match | PI3K pathway inhibitor | Responsive | BRCA | Late trials |
| PIK3CA | R93W | Complete Match | PIK3CA inhibitor | Responsive | BRCA | Early trials |
| PIK3CA | R93W | Complete Match | PIK3CA inhibitor | Responsive | ST | Case report |
| PIK3CA | R93W | Complete Match | AKT inhibitor | Responsive | BRCA | Early trials |
| PIK3CA | R93W | Complete Match | PI3K pathway inhibitor | Responsive;Responsive | L | Pre-clinical;Case report |
| PIK3CA | R93W | Complete Match | Cetuximab (EGFR mAb inhibitor) | Resistant | COREAD | Late trials |
| PIK3CA | R93W | Different Alteration | PI3K pathway inhibitor | Resistant | BRCA | Pre-clinical |
| PIK3CA | R88Q | Different Mutation | BRAF inhibitor | Resistant | CM | Case report |
| PIK3CA | R88Q | Complete Match | PI3K pathway inhibitor | Responsive | BLCA, HNC | Case report |
| PIK3CA | R88Q | Complete Match | PI3K pathway inhibitor | Responsive | HNSC | Case report |
| PIK3CA | R88Q | Complete Match | PI3K pathway inhibitor | Responsive | G, THCA | Pre-clinical |
| PIK3CA | R88Q | Complete Match | Everolimus;Trastuzumab;Chemotherapy (MTOR inhibitor;ERBB2 mAb inhibitor;Chemotherapy) | Responsive | BRCA | Late trials |
| PIK3CA | R88Q | Complete Match | PI3K pathway inhibitor | Responsive | COREAD | Pre-clinical |
| PIK3CA | R88Q | Complete Match | PI3K pathway inhibitor | Responsive | ED, OV, CESC | Early trials |
| PIK3CA | R88Q | Complete Match | PI3K pathway inhibitor | Responsive | BRCA | Late trials |
| PIK3CA | R88Q | Complete Match | PIK3CA inhibitor | Responsive | BRCA | Early trials |
| PIK3CA | R88Q | Complete Match | PIK3CA inhibitor | Responsive | ST | Case report |
| PIK3CA | R88Q | Complete Match | AKT inhibitor | Responsive | BRCA | Early trials |
| PIK3CA | R88Q | Complete Match | PI3K pathway inhibitor | Responsive;Responsive | L | Pre-clinical;Case report |
| PIK3CA | R88Q | Complete Match | Cetuximab (EGFR mAb inhibitor) | Resistant | COREAD | Late trials |
| PIK3CA | R88Q | Different Alteration | PI3K pathway inhibitor | Resistant | BRCA | Pre-clinical |
| PIK3CA | K111E | Different Mutation | BRAF inhibitor | Resistant | CM | Case report |
| PIK3CA | K111E | Complete Match | PI3K pathway inhibitor | Responsive | BLCA, HNC | Case report |
| PIK3CA | K111E | Complete Match | PI3K pathway inhibitor | Responsive | HNSC | Case report |
| PIK3CA | K111E | Complete Match | PI3K pathway inhibitor | Responsive | G, THCA | Pre-clinical |
| PIK3CA | K111E | Complete Match | Everolimus;Trastuzumab;Chemotherapy (MTOR inhibitor;ERBB2 mAb inhibitor;Chemotherapy) | Responsive | BRCA | Late trials |
| PIK3CA | K111E | Complete Match | PI3K pathway inhibitor | Responsive | COREAD | Pre-clinical |
| PIK3CA | K111E | Complete Match | PI3K pathway inhibitor | Responsive | ED, OV, CESC | Early trials |
| PIK3CA | K111E | Complete Match | PI3K pathway inhibitor | Responsive | BRCA | Late trials |
| PIK3CA | K111E | Complete Match | PIK3CA inhibitor | Responsive | BRCA | Early trials |
| PIK3CA | K111E | Complete Match | PIK3CA inhibitor | Responsive | ST | Case report |
| PIK3CA | K111E | Complete Match | AKT inhibitor | Responsive | BRCA | Early trials |
| PIK3CA | K111E | Complete Match | PI3K pathway inhibitor | Responsive;Responsive | L | Pre-clinical;Case report |
| PIK3CA | K111E | Complete Match | Cetuximab (EGFR mAb inhibitor) | Resistant | COREAD | Late trials |
| PIK3CA | K111E | Different Alteration | PI3K pathway inhibitor | Resistant | BRCA | Pre-clinical |
| PIK3CA | Q546R | Different Mutation | BRAF inhibitor | Resistant | CM | Case report |
| PIK3CA | Q546R | Complete Match | PI3K pathway inhibitor | Responsive | BLCA, HNC | Case report |
| PIK3CA | Q546R | Complete Match | PI3K pathway inhibitor | Responsive | HNSC | Case report |
| PIK3CA | Q546R | Complete Match | PI3K pathway inhibitor | Responsive | G, THCA | Pre-clinical |
| PIK3CA | Q546R | Complete Match | Everolimus;Trastuzumab;Chemotherapy (MTOR inhibitor;ERBB2 mAb inhibitor;Chemotherapy) | Responsive | BRCA | Late trials |
| PIK3CA | Q546R | Complete Match | PI3K pathway inhibitor | Responsive | COREAD | Pre-clinical |
| PIK3CA | Q546R | Complete Match | PI3K pathway inhibitor | Responsive | ED, OV, CESC | Early trials |
| PIK3CA | Q546R | Complete Match | PI3K pathway inhibitor | Responsive | BRCA | Late trials |
| PIK3CA | Q546R | Complete Match | PIK3CA inhibitor | Responsive | BRCA | Early trials |
| PIK3CA | Q546R | Complete Match | PIK3CA inhibitor | Responsive | ST | Case report |
| PIK3CA | Q546R | Complete Match | AKT inhibitor | Responsive | BRCA | Early trials |
| PIK3CA | Q546R | Complete Match | PI3K pathway inhibitor | Responsive;Responsive | L | Pre-clinical;Case report |
| PIK3CA | Q546R | Complete Match | Cetuximab (EGFR mAb inhibitor) | Resistant | COREAD | Late trials |
| PIK3CA | Q546R | Different Alteration | PI3K pathway inhibitor | Resistant | BRCA | Pre-clinical |
| PML | intron_variant | Different Alteration | Volasertib (PLK1 inhibitor) | Responsive | AML | Early trials |
| PML | intron_variant | Different Alteration | Tretinoin (Retinoid) | Responsive | APML | FDA guidelines |
| PML | intron_variant | Different Alteration | Tretinoin;Arsenic Trioxide (Retinoid;Chemotherapy) | Responsive | AML | FDA guidelines |
| PML | P447P | Different Alteration | Volasertib (PLK1 inhibitor) | Responsive | AML | Early trials |
| PML | P447P | Different Alteration | Tretinoin (Retinoid) | Responsive | APML | FDA guidelines |
| PML | P447P | Different Alteration | Tretinoin;Arsenic Trioxide (Retinoid;Chemotherapy) | Responsive | AML | FDA guidelines |
| PML | A273T), PML MUT* (intron_variant), PML MUT* (intron_variant | Different Alteration | Volasertib (PLK1 inhibitor) | Responsive | AML | Early trials |
| PML | A273T), PML MUT* (intron_variant), PML MUT* (intron_variant | Different Alteration | Tretinoin (Retinoid) | Responsive | APML | FDA guidelines |
| PML | A273T), PML MUT* (intron_variant), PML MUT* (intron_variant | Different Alteration | Tretinoin;Arsenic Trioxide (Retinoid;Chemotherapy) | Responsive | AML | FDA guidelines |
| PML | intron_variant), PML MUT* (intron_variant | Different Alteration | Volasertib (PLK1 inhibitor) | Responsive | AML | Early trials |
| PML | intron_variant), PML MUT* (intron_variant | Different Alteration | Tretinoin (Retinoid) | Responsive | APML | FDA guidelines |
| PML | intron_variant), PML MUT* (intron_variant | Different Alteration | Tretinoin;Arsenic Trioxide (Retinoid;Chemotherapy) | Responsive | AML | FDA guidelines |
| PML | G873C), PML MUT* (intron_variant), PML MUT* (IntronicBlockSubstitution | Different Alteration | Volasertib (PLK1 inhibitor) | Responsive | AML | Early trials |
| PML | G873C), PML MUT* (intron_variant), PML MUT* (IntronicBlockSubstitution | Different Alteration | Tretinoin (Retinoid) | Responsive | APML | FDA guidelines |
| PML | G873C), PML MUT* (intron_variant), PML MUT* (IntronicBlockSubstitution | Different Alteration | Tretinoin;Arsenic Trioxide (Retinoid;Chemotherapy) | Responsive | AML | FDA guidelines |
| PML | Y455Y), PML MUT* (intron_variant | Different Alteration | Volasertib (PLK1 inhibitor) | Responsive | AML | Early trials |
| PML | Y455Y), PML MUT* (intron_variant | Different Alteration | Tretinoin (Retinoid) | Responsive | APML | FDA guidelines |
| PML | Y455Y), PML MUT* (intron_variant | Different Alteration | Tretinoin;Arsenic Trioxide (Retinoid;Chemotherapy) | Responsive | AML | FDA guidelines |
| PML | 3-UTRSNV | Different Alteration | Volasertib (PLK1 inhibitor) | Responsive | AML | Early trials |
| PML | 3-UTRSNV | Different Alteration | Tretinoin (Retinoid) | Responsive | APML | FDA guidelines |
| PML | 3-UTRSNV | Different Alteration | Tretinoin;Arsenic Trioxide (Retinoid;Chemotherapy) | Responsive | AML | FDA guidelines |
| POLE | R150* | Complete Match | PD1/PDL1 inhibitor | Responsive | UTH | Early trials |
| POLE | R150* | Complete Match | PD1 Ab inhibitor | Responsive | COREAD | Case report |
| POLE | R150* | Different Mutation | PD1 Ab inhibitor | Responsive | G, ED | Case report |
| POLE | R150* | Different Alteration | PD1/PDL1 inhibitor | Responsive | UTH | Early trials |
| PTEN | splice_donor_variant | Different Mutation | Cetuximab (EGFR mAb inhibitor) | Resistant | COREAD | Case report |
| PTEN | splice_donor_variant | Different Mutation | Panitumumab (EGFR mAb inhibitor) | Resistant | COREAD | Case report |
| PTEN | splice_donor_variant | Different Mutation | BYL719 (PIK3CA inhibitor) | Resistant | BRCA | Case report |
| PTEN | splice_donor_variant | Complete Match | ATM inhibitor | Responsive | BRCA | Pre-clinical |
| PTEN | splice_donor_variant | Complete Match | PI3K pathway inhibitor | Responsive | BRCA, ED, TH, CANCER, L, G, OV | Pre-clinical |
| PTEN | splice_donor_variant | Complete Match | EGFR mAb inhibitor | Resistant | COREAD | Late trials |
| PTEN | splice_donor_variant | Complete Match | Sirolimus (MTOR inhibitor) | Responsive | CANCER | Early trials |
| PTEN | splice_donor_variant | Complete Match | PARP inhibitor | Responsive | ED | Case report |
| PTEN | splice_donor_variant | Complete Match | PD1 Ab inhibitor | Resistant | CM | Early trials |
| PTEN | splice_donor_variant | Complete Match | PI3K pathway inhibitor;AR antagonist | Responsive | PRAD | Pre-clinical |
| PTEN | splice_donor_variant | Complete Match | Everolimus (MTOR inhibitor) | Responsive | PRAD | Early trials |
| PTEN | splice_donor_variant | Complete Match | PI3K pathway inhibitor;MEK inhibitor | Responsive | OV | Pre-clinical |
| PTEN | splice_donor_variant | Complete Match | PIK3CB inhibitor | Responsive | PRAD | Case report |
| PTEN | splice_donor_variant | Complete Match | AKT inhibitor | Responsive | PA | Case report |
| PTEN | splice_donor_variant | Complete Match | MTOR inhibitor | No Responsive | ED | Early trials |
| PTEN | splice_donor_variant | Different Alteration | PI3K pathway inhibitor | Responsive | L, BRCA, CANCER, G, ED, TH, OV | Pre-clinical |
| PTEN | splice_donor_variant | Different Alteration | Everolimus (MTOR inhibitor) | Responsive | PRAD | Early trials |
| PTEN | splice_donor_variant | Different Alteration | Sirolimus (MTOR inhibitor) | Responsive | CANCER | Early trials |
| PTEN | splice_donor_variant | Different Alteration | PARP inhibitor | Responsive | ED | Case report |
| PTEN | splice_donor_variant | Different Alteration | PIK3CB inhibitor | Responsive | PRAD | Case report |
| PTEN | splice_donor_variant | Different Alteration | EGFR mAb inhibitor | Resistant | COREAD | Late trials |
| PTEN | splice_donor_variant | Different Alteration | MTOR inhibitor | No Responsive | ED | Early trials |
| PTEN | splice_donor_variant | Different Alteration | AKT inhibitor | Responsive | PA | Case report |
| PTEN | splice_donor_variant | Different Alteration | PI3K pathway inhibitor;AR antagonist | Responsive | PRAD | Pre-clinical |
| PTEN | splice_donor_variant | Different Alteration | PI3K pathway inhibitor;MEK inhibitor | Responsive | OV | Pre-clinical |
| PTEN | R130Q), PTEN MUT (E43* | Complete Match | Cetuximab (EGFR mAb inhibitor) | Resistant | COREAD | Case report |
| PTEN | R130Q), PTEN MUT (E43* | Complete Match | Panitumumab (EGFR mAb inhibitor) | Resistant | COREAD | Case report |
| PTEN | R130Q), PTEN MUT (E43* | Complete Match | BYL719 (PIK3CA inhibitor) | Resistant | BRCA | Case report |
| PTEN | R130Q), PTEN MUT (E43* | Complete Match | ATM inhibitor | Responsive | BRCA | Pre-clinical |
| PTEN | R130Q), PTEN MUT (E43* | Complete Match | PI3K pathway inhibitor | Responsive | BRCA, ED, TH, CANCER, L, G, OV | Pre-clinical |
| PTEN | R130Q), PTEN MUT (E43* | Complete Match | EGFR mAb inhibitor | Resistant | COREAD | Late trials |
| PTEN | R130Q), PTEN MUT (E43* | Complete Match | Sirolimus (MTOR inhibitor) | Responsive | CANCER | Early trials |
| PTEN | R130Q), PTEN MUT (E43* | Complete Match | PARP inhibitor | Responsive | ED | Case report |
| PTEN | R130Q), PTEN MUT (E43* | Complete Match | PD1 Ab inhibitor | Resistant | CM | Early trials |
| PTEN | R130Q), PTEN MUT (E43* | Complete Match | PI3K pathway inhibitor;AR antagonist | Responsive | PRAD | Pre-clinical |
| PTEN | R130Q), PTEN MUT (E43* | Complete Match | Everolimus (MTOR inhibitor) | Responsive | PRAD | Early trials |
| PTEN | R130Q), PTEN MUT (E43* | Complete Match | PI3K pathway inhibitor;MEK inhibitor | Responsive | OV | Pre-clinical |
| PTEN | R130Q), PTEN MUT (E43* | Complete Match | PIK3CB inhibitor | Responsive | PRAD | Case report |
| PTEN | R130Q), PTEN MUT (E43* | Complete Match | AKT inhibitor | Responsive | PA | Case report |
| PTEN | R130Q), PTEN MUT (E43* | Complete Match | MTOR inhibitor | No Responsive | ED | Early trials |
| PTEN | R130Q), PTEN MUT (E43* | Different Alteration | PI3K pathway inhibitor | Responsive | L, BRCA, CANCER, G, ED, TH, OV | Pre-clinical |
| PTEN | R130Q), PTEN MUT (E43* | Different Alteration | Everolimus (MTOR inhibitor) | Responsive | PRAD | Early trials |
| PTEN | R130Q), PTEN MUT (E43* | Different Alteration | Sirolimus (MTOR inhibitor) | Responsive | CANCER | Early trials |
| PTEN | R130Q), PTEN MUT (E43* | Different Alteration | PARP inhibitor | Responsive | ED | Case report |
| PTEN | R130Q), PTEN MUT (E43* | Different Alteration | PIK3CB inhibitor | Responsive | PRAD | Case report |
| PTEN | R130Q), PTEN MUT (E43* | Different Alteration | EGFR mAb inhibitor | Resistant | COREAD | Late trials |
| PTEN | R130Q), PTEN MUT (E43* | Different Alteration | MTOR inhibitor | No Responsive | ED | Early trials |
| PTEN | R130Q), PTEN MUT (E43* | Different Alteration | AKT inhibitor | Responsive | PA | Case report |
| PTEN | R130Q), PTEN MUT (E43* | Different Alteration | PI3K pathway inhibitor;AR antagonist | Responsive | PRAD | Pre-clinical |
| PTEN | R130Q), PTEN MUT (E43* | Different Alteration | PI3K pathway inhibitor;MEK inhibitor | Responsive | OV | Pre-clinical |
| RAF1 | intron_variant), RAF1 MUT* (intron_variant | Different Alteration | Sorafenib (Pan-TK inhibitor) | Responsive | PRAD | Pre-clinical |
| RAF1 | intron_variant), RAF1 MUT* (intron_variant | Different Alteration | Pan-RAF inhibitor | Responsive | PRAD | Pre-clinical |
| RAF1 | intron_variant), RAF1 MUT* (intron_variant | Different Alteration | U0126 (MEK inhibitor) | Responsive | PRAD | Pre-clinical |
| RAF1 | intron_variant | Different Alteration | Sorafenib (Pan-TK inhibitor) | Responsive | PRAD | Pre-clinical |
| RAF1 | intron_variant | Different Alteration | Pan-RAF inhibitor | Responsive | PRAD | Pre-clinical |
| RAF1 | intron_variant | Different Alteration | U0126 (MEK inhibitor) | Responsive | PRAD | Pre-clinical |
| RAF1 | intron_variant), RAF1 MUT* (intron_variant), RAF1 MUT* (intron_variant | Different Alteration | Sorafenib (Pan-TK inhibitor) | Responsive | PRAD | Pre-clinical |
| RAF1 | intron_variant), RAF1 MUT* (intron_variant), RAF1 MUT* (intron_variant | Different Alteration | Pan-RAF inhibitor | Responsive | PRAD | Pre-clinical |
| RAF1 | intron_variant), RAF1 MUT* (intron_variant), RAF1 MUT* (intron_variant | Different Alteration | U0126 (MEK inhibitor) | Responsive | PRAD | Pre-clinical |
| RAF1 | V492L | Different Alteration | Sorafenib (Pan-TK inhibitor) | Responsive | PRAD | Pre-clinical |
| RAF1 | V492L | Different Alteration | Pan-RAF inhibitor | Responsive | PRAD | Pre-clinical |
| RAF1 | V492L | Different Alteration | U0126 (MEK inhibitor) | Responsive | PRAD | Pre-clinical |
| RB1 | splice_donor_variant | Different Alteration | Palbociclib (CDK4/6 inhibitor) | Responsive | PRAD | Pre-clinical |
| RB1 | splice_donor_variant | Complete Match | MDM2/MDMX inhibitor | Responsive | RB | Pre-clinical |
| RB1 | splice_donor_variant | Complete Match | Cisplatin (Chemotherapy) | Responsive | BLCA | Early trials |
| RB1 | splice_donor_variant | Complete Match | HDAC inhibitor | Responsive | RB | Pre-clinical |
| RB1 | splice_donor_variant | Different Alteration | MDM2/MDMX inhibitor | Responsive | RB | Pre-clinical |
| RB1 | splice_donor_variant | Different Alteration | Cisplatin (Chemotherapy) | Responsive | BLCA | Early trials |
| RB1 | splice_donor_variant | Different Alteration | HDAC inhibitor | Responsive | RB | Pre-clinical |
| RB1 | K341* | Different Alteration | Palbociclib (CDK4/6 inhibitor) | Responsive | PRAD | Pre-clinical |
| RB1 | K341* | Complete Match | MDM2/MDMX inhibitor | Responsive | RB | Pre-clinical |
| RB1 | K341* | Complete Match | Cisplatin (Chemotherapy) | Responsive | BLCA | Early trials |
| RB1 | K341* | Complete Match | HDAC inhibitor | Responsive | RB | Pre-clinical |
| RB1 | K341* | Different Alteration | MDM2/MDMX inhibitor | Responsive | RB | Pre-clinical |
| RB1 | K341* | Different Alteration | Cisplatin (Chemotherapy) | Responsive | BLCA | Early trials |
| RB1 | K341* | Different Alteration | HDAC inhibitor | Responsive | RB | Pre-clinical |
| RET | A640D | Different Alteration | Nintedanib (Pan-TK inhibitor) | Responsive | LUAD | Case report |
| RET | A640D | Different Alteration | Cabozantinib (Pan-kinase inhibitor) | Responsive | LUAD | Early trials |
| RET | A640D | Different Alteration | RET inhibitor | Responsive | LUAD | Pre-clinical |
| RET | A640D | Different Alteration | Vandetanib (Pan-TK inhibitor) | Responsive | LUAD | Early trials |
| RET | A640D | Different Alteration | Sunitinib (Pan-TK inhibitor) | Responsive | LUAD | Early trials |
| RET | A640D | Different Alteration | Cabozantinib (Pan-kinase inhibitor) | Responsive | THCA | Pre-clinical |
| RET | A640D | Different Alteration | Vandetanib (Pan-TK inhibitor) | Responsive | THCA | Pre-clinical |
| RET | A640D | Different Alteration | Sunitinib (Pan-TK inhibitor) | Responsive | THCA | Pre-clinical |
| RET | A640D | Different Alteration | RET inhibitor | Responsive | TH | Pre-clinical |
| RET | intron_variant | Different Alteration | Nintedanib (Pan-TK inhibitor) | Responsive | LUAD | Case report |
| RET | intron_variant | Different Alteration | Cabozantinib (Pan-kinase inhibitor) | Responsive | LUAD | Early trials |
| RET | intron_variant | Different Alteration | RET inhibitor | Responsive | LUAD | Pre-clinical |
| RET | intron_variant | Different Alteration | Vandetanib (Pan-TK inhibitor) | Responsive | LUAD | Early trials |
| RET | intron_variant | Different Alteration | Sunitinib (Pan-TK inhibitor) | Responsive | LUAD | Early trials |
| RET | intron_variant | Different Alteration | Cabozantinib (Pan-kinase inhibitor) | Responsive | THCA | Pre-clinical |
| RET | intron_variant | Different Alteration | Vandetanib (Pan-TK inhibitor) | Responsive | THCA | Pre-clinical |
| RET | intron_variant | Different Alteration | Sunitinib (Pan-TK inhibitor) | Responsive | THCA | Pre-clinical |
| RET | intron_variant | Different Alteration | RET inhibitor | Responsive | TH | Pre-clinical |
| RET | R133C), RET MUT* (intron_variant | Different Alteration | Nintedanib (Pan-TK inhibitor) | Responsive | LUAD | Case report |
| RET | R133C), RET MUT* (intron_variant | Different Alteration | Cabozantinib (Pan-kinase inhibitor) | Responsive | LUAD | Early trials |
| RET | R133C), RET MUT* (intron_variant | Different Alteration | RET inhibitor | Responsive | LUAD | Pre-clinical |
| RET | R133C), RET MUT* (intron_variant | Different Alteration | Vandetanib (Pan-TK inhibitor) | Responsive | LUAD | Early trials |
| RET | R133C), RET MUT* (intron_variant | Different Alteration | Sunitinib (Pan-TK inhibitor) | Responsive | LUAD | Early trials |
| RET | R133C), RET MUT* (intron_variant | Different Alteration | Cabozantinib (Pan-kinase inhibitor) | Responsive | THCA | Pre-clinical |
| RET | R133C), RET MUT* (intron_variant | Different Alteration | Vandetanib (Pan-TK inhibitor) | Responsive | THCA | Pre-clinical |
| RET | R133C), RET MUT* (intron_variant | Different Alteration | Sunitinib (Pan-TK inhibitor) | Responsive | THCA | Pre-clinical |
| RET | R133C), RET MUT* (intron_variant | Different Alteration | RET inhibitor | Responsive | TH | Pre-clinical |
| RET | S891L), RET MUT* (intron_variant | Different Alteration | Nintedanib (Pan-TK inhibitor) | Responsive | LUAD | Case report |
| RET | S891L), RET MUT* (intron_variant | Different Alteration | Cabozantinib (Pan-kinase inhibitor) | Responsive | LUAD | Early trials |
| RET | S891L), RET MUT* (intron_variant | Different Alteration | RET inhibitor | Responsive | LUAD | Pre-clinical |
| RET | S891L), RET MUT* (intron_variant | Different Alteration | Vandetanib (Pan-TK inhibitor) | Responsive | LUAD | Early trials |
| RET | S891L), RET MUT* (intron_variant | Different Alteration | Sunitinib (Pan-TK inhibitor) | Responsive | LUAD | Early trials |
| RET | S891L), RET MUT* (intron_variant | Different Alteration | Cabozantinib (Pan-kinase inhibitor) | Responsive | THCA | Pre-clinical |
| RET | S891L), RET MUT* (intron_variant | Different Alteration | Vandetanib (Pan-TK inhibitor) | Responsive | THCA | Pre-clinical |
| RET | S891L), RET MUT* (intron_variant | Different Alteration | Sunitinib (Pan-TK inhibitor) | Responsive | THCA | Pre-clinical |
| RET | S891L), RET MUT* (intron_variant | Different Alteration | RET inhibitor | Responsive | TH | Pre-clinical |
| RET | intron_variant), RET MUT* (intron_variant), RET MUT* (intron_variant | Different Alteration | Nintedanib (Pan-TK inhibitor) | Responsive | LUAD | Case report |
| RET | intron_variant), RET MUT* (intron_variant), RET MUT* (intron_variant | Different Alteration | Cabozantinib (Pan-kinase inhibitor) | Responsive | LUAD | Early trials |
| RET | intron_variant), RET MUT* (intron_variant), RET MUT* (intron_variant | Different Alteration | RET inhibitor | Responsive | LUAD | Pre-clinical |
| RET | intron_variant), RET MUT* (intron_variant), RET MUT* (intron_variant | Different Alteration | Vandetanib (Pan-TK inhibitor) | Responsive | LUAD | Early trials |
| RET | intron_variant), RET MUT* (intron_variant), RET MUT* (intron_variant | Different Alteration | Sunitinib (Pan-TK inhibitor) | Responsive | LUAD | Early trials |
| RET | intron_variant), RET MUT* (intron_variant), RET MUT* (intron_variant | Different Alteration | Cabozantinib (Pan-kinase inhibitor) | Responsive | THCA | Pre-clinical |
| RET | intron_variant), RET MUT* (intron_variant), RET MUT* (intron_variant | Different Alteration | Vandetanib (Pan-TK inhibitor) | Responsive | THCA | Pre-clinical |
| RET | intron_variant), RET MUT* (intron_variant), RET MUT* (intron_variant | Different Alteration | Sunitinib (Pan-TK inhibitor) | Responsive | THCA | Pre-clinical |
| RET | intron_variant), RET MUT* (intron_variant), RET MUT* (intron_variant | Different Alteration | RET inhibitor | Responsive | TH | Pre-clinical |
| RET | A513V | Different Alteration | Nintedanib (Pan-TK inhibitor) | Responsive | LUAD | Case report |
| RET | A513V | Different Alteration | Cabozantinib (Pan-kinase inhibitor) | Responsive | LUAD | Early trials |
| RET | A513V | Different Alteration | RET inhibitor | Responsive | LUAD | Pre-clinical |
| RET | A513V | Different Alteration | Vandetanib (Pan-TK inhibitor) | Responsive | LUAD | Early trials |
| RET | A513V | Different Alteration | Sunitinib (Pan-TK inhibitor) | Responsive | LUAD | Early trials |
| RET | A513V | Different Alteration | Cabozantinib (Pan-kinase inhibitor) | Responsive | THCA | Pre-clinical |
| RET | A513V | Different Alteration | Vandetanib (Pan-TK inhibitor) | Responsive | THCA | Pre-clinical |
| RET | A513V | Different Alteration | Sunitinib (Pan-TK inhibitor) | Responsive | THCA | Pre-clinical |
| RET | A513V | Different Alteration | RET inhibitor | Responsive | TH | Pre-clinical |
| RET | R600W | Different Alteration | Nintedanib (Pan-TK inhibitor) | Responsive | LUAD | Case report |
| RET | R600W | Different Alteration | Cabozantinib (Pan-kinase inhibitor) | Responsive | LUAD | Early trials |
| RET | R600W | Different Alteration | RET inhibitor | Responsive | LUAD | Pre-clinical |
| RET | R600W | Different Alteration | Vandetanib (Pan-TK inhibitor) | Responsive | LUAD | Early trials |
| RET | R600W | Different Alteration | Sunitinib (Pan-TK inhibitor) | Responsive | LUAD | Early trials |
| RET | R600W | Different Alteration | Cabozantinib (Pan-kinase inhibitor) | Responsive | THCA | Pre-clinical |
| RET | R600W | Different Alteration | Vandetanib (Pan-TK inhibitor) | Responsive | THCA | Pre-clinical |
| RET | R600W | Different Alteration | Sunitinib (Pan-TK inhibitor) | Responsive | THCA | Pre-clinical |
| RET | R600W | Different Alteration | RET inhibitor | Responsive | TH | Pre-clinical |
| RET | 3-UTRSNV | Different Alteration | Nintedanib (Pan-TK inhibitor) | Responsive | LUAD | Case report |
| RET | 3-UTRSNV | Different Alteration | Cabozantinib (Pan-kinase inhibitor) | Responsive | LUAD | Early trials |
| RET | 3-UTRSNV | Different Alteration | RET inhibitor | Responsive | LUAD | Pre-clinical |
| RET | 3-UTRSNV | Different Alteration | Vandetanib (Pan-TK inhibitor) | Responsive | LUAD | Early trials |
| RET | 3-UTRSNV | Different Alteration | Sunitinib (Pan-TK inhibitor) | Responsive | LUAD | Early trials |
| RET | 3-UTRSNV | Different Alteration | Cabozantinib (Pan-kinase inhibitor) | Responsive | THCA | Pre-clinical |
| RET | 3-UTRSNV | Different Alteration | Vandetanib (Pan-TK inhibitor) | Responsive | THCA | Pre-clinical |
| RET | 3-UTRSNV | Different Alteration | Sunitinib (Pan-TK inhibitor) | Responsive | THCA | Pre-clinical |
| RET | 3-UTRSNV | Different Alteration | RET inhibitor | Responsive | TH | Pre-clinical |
| RET | S765S), RET MUT* (intron_variant | Different Alteration | Nintedanib (Pan-TK inhibitor) | Responsive | LUAD | Case report |
| RET | S765S), RET MUT* (intron_variant | Different Alteration | Cabozantinib (Pan-kinase inhibitor) | Responsive | LUAD | Early trials |
| RET | S765S), RET MUT* (intron_variant | Different Alteration | RET inhibitor | Responsive | LUAD | Pre-clinical |
| RET | S765S), RET MUT* (intron_variant | Different Alteration | Vandetanib (Pan-TK inhibitor) | Responsive | LUAD | Early trials |
| RET | S765S), RET MUT* (intron_variant | Different Alteration | Sunitinib (Pan-TK inhibitor) | Responsive | LUAD | Early trials |
| RET | S765S), RET MUT* (intron_variant | Different Alteration | Cabozantinib (Pan-kinase inhibitor) | Responsive | THCA | Pre-clinical |
| RET | S765S), RET MUT* (intron_variant | Different Alteration | Vandetanib (Pan-TK inhibitor) | Responsive | THCA | Pre-clinical |
| RET | S765S), RET MUT* (intron_variant | Different Alteration | Sunitinib (Pan-TK inhibitor) | Responsive | THCA | Pre-clinical |
| RET | S765S), RET MUT* (intron_variant | Different Alteration | RET inhibitor | Responsive | TH | Pre-clinical |
| RET | intron_variant), RET MUT* (intron_variant | Different Alteration | Nintedanib (Pan-TK inhibitor) | Responsive | LUAD | Case report |
| RET | intron_variant), RET MUT* (intron_variant | Different Alteration | Cabozantinib (Pan-kinase inhibitor) | Responsive | LUAD | Early trials |
| RET | intron_variant), RET MUT* (intron_variant | Different Alteration | RET inhibitor | Responsive | LUAD | Pre-clinical |
| RET | intron_variant), RET MUT* (intron_variant | Different Alteration | Vandetanib (Pan-TK inhibitor) | Responsive | LUAD | Early trials |
| RET | intron_variant), RET MUT* (intron_variant | Different Alteration | Sunitinib (Pan-TK inhibitor) | Responsive | LUAD | Early trials |
| RET | intron_variant), RET MUT* (intron_variant | Different Alteration | Cabozantinib (Pan-kinase inhibitor) | Responsive | THCA | Pre-clinical |
| RET | intron_variant), RET MUT* (intron_variant | Different Alteration | Vandetanib (Pan-TK inhibitor) | Responsive | THCA | Pre-clinical |
| RET | intron_variant), RET MUT* (intron_variant | Different Alteration | Sunitinib (Pan-TK inhibitor) | Responsive | THCA | Pre-clinical |
| RET | intron_variant), RET MUT* (intron_variant | Different Alteration | RET inhibitor | Responsive | TH | Pre-clinical |
| RET | A472G), RET MUT* (intron_variant | Different Alteration | Nintedanib (Pan-TK inhibitor) | Responsive | LUAD | Case report |
| RET | A472G), RET MUT* (intron_variant | Different Alteration | Cabozantinib (Pan-kinase inhibitor) | Responsive | LUAD | Early trials |
| RET | A472G), RET MUT* (intron_variant | Different Alteration | RET inhibitor | Responsive | LUAD | Pre-clinical |
| RET | A472G), RET MUT* (intron_variant | Different Alteration | Vandetanib (Pan-TK inhibitor) | Responsive | LUAD | Early trials |
| RET | A472G), RET MUT* (intron_variant | Different Alteration | Sunitinib (Pan-TK inhibitor) | Responsive | LUAD | Early trials |
| RET | A472G), RET MUT* (intron_variant | Different Alteration | Cabozantinib (Pan-kinase inhibitor) | Responsive | THCA | Pre-clinical |
| RET | A472G), RET MUT* (intron_variant | Different Alteration | Vandetanib (Pan-TK inhibitor) | Responsive | THCA | Pre-clinical |
| RET | A472G), RET MUT* (intron_variant | Different Alteration | Sunitinib (Pan-TK inhibitor) | Responsive | THCA | Pre-clinical |
| RET | A472G), RET MUT* (intron_variant | Different Alteration | RET inhibitor | Responsive | TH | Pre-clinical |
| RET | IntronicBlockSubstitution | Different Alteration | Nintedanib (Pan-TK inhibitor) | Responsive | LUAD | Case report |
| RET | IntronicBlockSubstitution | Different Alteration | Cabozantinib (Pan-kinase inhibitor) | Responsive | LUAD | Early trials |
| RET | IntronicBlockSubstitution | Different Alteration | RET inhibitor | Responsive | LUAD | Pre-clinical |
| RET | IntronicBlockSubstitution | Different Alteration | Vandetanib (Pan-TK inhibitor) | Responsive | LUAD | Early trials |
| RET | IntronicBlockSubstitution | Different Alteration | Sunitinib (Pan-TK inhibitor) | Responsive | LUAD | Early trials |
| RET | IntronicBlockSubstitution | Different Alteration | Cabozantinib (Pan-kinase inhibitor) | Responsive | THCA | Pre-clinical |
| RET | IntronicBlockSubstitution | Different Alteration | Vandetanib (Pan-TK inhibitor) | Responsive | THCA | Pre-clinical |
| RET | IntronicBlockSubstitution | Different Alteration | Sunitinib (Pan-TK inhibitor) | Responsive | THCA | Pre-clinical |
| RET | IntronicBlockSubstitution | Different Alteration | RET inhibitor | Responsive | TH | Pre-clinical |
| RNF43 | splice_acceptor_variant | Complete Match | Porcupine inhibitor | Responsive | COREAD | Case report |
| RNF43 | splice_acceptor_variant), RNF43 MUT (Q153* | Complete Match | Porcupine inhibitor | Responsive | COREAD | Case report |
| ROS1 | intron_variant | Different Alteration | Crizotinib (ALK inhibitor) | Responsive | IM | Case report |
| ROS1 | intron_variant | Different Alteration | Crizotinib (ALK inhibitor) | Responsive | NSCLC | FDA guidelines |
| ROS1 | intron_variant | Different Alteration | Cabozantinib (Pan-kinase inhibitor) | Responsive | LUAD | Case report |
| ROS1 | intron_variant | Different Alteration | HSP90 inhibitor | Responsive | LUAD | Pre-clinical |
| ROS1 | intron_variant | Different Alteration | Crizotinib (ALK inhibitor) | Responsive | LUAD | Early trials |
| ROS1 | intron_variant), ROS1 MUT* (intron_variant | Different Alteration | Crizotinib (ALK inhibitor) | Responsive | IM | Case report |
| ROS1 | intron_variant), ROS1 MUT* (intron_variant | Different Alteration | Crizotinib (ALK inhibitor) | Responsive | NSCLC | FDA guidelines |
| ROS1 | intron_variant), ROS1 MUT* (intron_variant | Different Alteration | Cabozantinib (Pan-kinase inhibitor) | Responsive | LUAD | Case report |
| ROS1 | intron_variant), ROS1 MUT* (intron_variant | Different Alteration | HSP90 inhibitor | Responsive | LUAD | Pre-clinical |
| ROS1 | intron_variant), ROS1 MUT* (intron_variant | Different Alteration | Crizotinib (ALK inhibitor) | Responsive | LUAD | Early trials |
| ROS1 | 3-UTRSNV | Different Alteration | Crizotinib (ALK inhibitor) | Responsive | IM | Case report |
| ROS1 | 3-UTRSNV | Different Alteration | Crizotinib (ALK inhibitor) | Responsive | NSCLC | FDA guidelines |
| ROS1 | 3-UTRSNV | Different Alteration | Cabozantinib (Pan-kinase inhibitor) | Responsive | LUAD | Case report |
| ROS1 | 3-UTRSNV | Different Alteration | HSP90 inhibitor | Responsive | LUAD | Pre-clinical |
| ROS1 | 3-UTRSNV | Different Alteration | Crizotinib (ALK inhibitor) | Responsive | LUAD | Early trials |
| ROS1 | R771K | Different Alteration | Crizotinib (ALK inhibitor) | Responsive | IM | Case report |
| ROS1 | R771K | Different Alteration | Crizotinib (ALK inhibitor) | Responsive | NSCLC | FDA guidelines |
| ROS1 | R771K | Different Alteration | Cabozantinib (Pan-kinase inhibitor) | Responsive | LUAD | Case report |
| ROS1 | R771K | Different Alteration | HSP90 inhibitor | Responsive | LUAD | Pre-clinical |
| ROS1 | R771K | Different Alteration | Crizotinib (ALK inhibitor) | Responsive | LUAD | Early trials |
| ROS1 | D735V | Different Alteration | Crizotinib (ALK inhibitor) | Responsive | IM | Case report |
| ROS1 | D735V | Different Alteration | Crizotinib (ALK inhibitor) | Responsive | NSCLC | FDA guidelines |
| ROS1 | D735V | Different Alteration | Cabozantinib (Pan-kinase inhibitor) | Responsive | LUAD | Case report |
| ROS1 | D735V | Different Alteration | HSP90 inhibitor | Responsive | LUAD | Pre-clinical |
| ROS1 | D735V | Different Alteration | Crizotinib (ALK inhibitor) | Responsive | LUAD | Early trials |
| ROS1 | S283T | Different Alteration | Crizotinib (ALK inhibitor) | Responsive | IM | Case report |
| ROS1 | S283T | Different Alteration | Crizotinib (ALK inhibitor) | Responsive | NSCLC | FDA guidelines |
| ROS1 | S283T | Different Alteration | Cabozantinib (Pan-kinase inhibitor) | Responsive | LUAD | Case report |
| ROS1 | S283T | Different Alteration | HSP90 inhibitor | Responsive | LUAD | Pre-clinical |
| ROS1 | S283T | Different Alteration | Crizotinib (ALK inhibitor) | Responsive | LUAD | Early trials |
| ROS1 | intron_variant), ROS1 MUT* (intron_variant), ROS1 MUT* (intron_variant | Different Alteration | Crizotinib (ALK inhibitor) | Responsive | IM | Case report |
| ROS1 | intron_variant), ROS1 MUT* (intron_variant), ROS1 MUT* (intron_variant | Different Alteration | Crizotinib (ALK inhibitor) | Responsive | NSCLC | FDA guidelines |
| ROS1 | intron_variant), ROS1 MUT* (intron_variant), ROS1 MUT* (intron_variant | Different Alteration | Cabozantinib (Pan-kinase inhibitor) | Responsive | LUAD | Case report |
| ROS1 | intron_variant), ROS1 MUT* (intron_variant), ROS1 MUT* (intron_variant | Different Alteration | HSP90 inhibitor | Responsive | LUAD | Pre-clinical |
| ROS1 | intron_variant), ROS1 MUT* (intron_variant), ROS1 MUT* (intron_variant | Different Alteration | Crizotinib (ALK inhibitor) | Responsive | LUAD | Early trials |
| ROS1 | G2282G | Different Alteration | Crizotinib (ALK inhibitor) | Responsive | IM | Case report |
| ROS1 | G2282G | Different Alteration | Crizotinib (ALK inhibitor) | Responsive | NSCLC | FDA guidelines |
| ROS1 | G2282G | Different Alteration | Cabozantinib (Pan-kinase inhibitor) | Responsive | LUAD | Case report |
| ROS1 | G2282G | Different Alteration | HSP90 inhibitor | Responsive | LUAD | Pre-clinical |
| ROS1 | G2282G | Different Alteration | Crizotinib (ALK inhibitor) | Responsive | LUAD | Early trials |
| ROS1 | intron_variant), ROS1 MUT* (intron_variant), ROS1 MUT* (intron_variant), ROS1 MUT* (intron_variant), ROS1 MUT* (intron_variant), ROS1 MUT* (intron_variant), ROS1 MUT* (W1644*), ROS1 MUT* (intron_variant | Different Alteration | Crizotinib (ALK inhibitor) | Responsive | IM | Case report |
| ROS1 | intron_variant), ROS1 MUT* (intron_variant), ROS1 MUT* (intron_variant), ROS1 MUT* (intron_variant), ROS1 MUT* (intron_variant), ROS1 MUT* (intron_variant), ROS1 MUT* (W1644*), ROS1 MUT* (intron_variant | Different Alteration | Crizotinib (ALK inhibitor) | Responsive | NSCLC | FDA guidelines |
| ROS1 | intron_variant), ROS1 MUT* (intron_variant), ROS1 MUT* (intron_variant), ROS1 MUT* (intron_variant), ROS1 MUT* (intron_variant), ROS1 MUT* (intron_variant), ROS1 MUT* (W1644*), ROS1 MUT* (intron_variant | Different Alteration | Cabozantinib (Pan-kinase inhibitor) | Responsive | LUAD | Case report |
| ROS1 | intron_variant), ROS1 MUT* (intron_variant), ROS1 MUT* (intron_variant), ROS1 MUT* (intron_variant), ROS1 MUT* (intron_variant), ROS1 MUT* (intron_variant), ROS1 MUT* (W1644*), ROS1 MUT* (intron_variant | Different Alteration | HSP90 inhibitor | Responsive | LUAD | Pre-clinical |
| ROS1 | intron_variant), ROS1 MUT* (intron_variant), ROS1 MUT* (intron_variant), ROS1 MUT* (intron_variant), ROS1 MUT* (intron_variant), ROS1 MUT* (intron_variant), ROS1 MUT* (W1644*), ROS1 MUT* (intron_variant | Different Alteration | Crizotinib (ALK inhibitor) | Responsive | LUAD | Early trials |
| ROS1 | intron_variant), ROS1 MUT* (intron_variant), ROS1 MUT* (intron_variant), ROS1 MUT* (intron_variant | Different Alteration | Crizotinib (ALK inhibitor) | Responsive | IM | Case report |
| ROS1 | intron_variant), ROS1 MUT* (intron_variant), ROS1 MUT* (intron_variant), ROS1 MUT* (intron_variant | Different Alteration | Crizotinib (ALK inhibitor) | Responsive | NSCLC | FDA guidelines |
| ROS1 | intron_variant), ROS1 MUT* (intron_variant), ROS1 MUT* (intron_variant), ROS1 MUT* (intron_variant | Different Alteration | Cabozantinib (Pan-kinase inhibitor) | Responsive | LUAD | Case report |
| ROS1 | intron_variant), ROS1 MUT* (intron_variant), ROS1 MUT* (intron_variant), ROS1 MUT* (intron_variant | Different Alteration | HSP90 inhibitor | Responsive | LUAD | Pre-clinical |
| ROS1 | intron_variant), ROS1 MUT* (intron_variant), ROS1 MUT* (intron_variant), ROS1 MUT* (intron_variant | Different Alteration | Crizotinib (ALK inhibitor) | Responsive | LUAD | Early trials |
| ROS1 | intron_variant), ROS1 MUT* (Y1725Y | Different Alteration | Crizotinib (ALK inhibitor) | Responsive | IM | Case report |
| ROS1 | intron_variant), ROS1 MUT* (Y1725Y | Different Alteration | Crizotinib (ALK inhibitor) | Responsive | NSCLC | FDA guidelines |
| ROS1 | intron_variant), ROS1 MUT* (Y1725Y | Different Alteration | Cabozantinib (Pan-kinase inhibitor) | Responsive | LUAD | Case report |
| ROS1 | intron_variant), ROS1 MUT* (Y1725Y | Different Alteration | HSP90 inhibitor | Responsive | LUAD | Pre-clinical |
| ROS1 | intron_variant), ROS1 MUT* (Y1725Y | Different Alteration | Crizotinib (ALK inhibitor) | Responsive | LUAD | Early trials |
| SETD2 | R400* | Complete Match | WEE1 inhibitor | Responsive | CANCER | Pre-clinical |
| SETD2 | R400* | Different Alteration | WEE1 inhibitor | Responsive | CANCER | Pre-clinical |
| SETD2 | E2436* | Complete Match | WEE1 inhibitor | Responsive | CANCER | Pre-clinical |
| SETD2 | E2436* | Different Alteration | WEE1 inhibitor | Responsive | CANCER | Pre-clinical |
| SF3B1 | K700E | Complete Match | Spliceosome inhibitor | Responsive | CANCER | Pre-clinical |
| SF3B1 | R625C | Different Mutation | Spliceosome inhibitor | Responsive | CANCER | Pre-clinical |
| STAG2 | R1012* | Complete Match | PARP inhibitor | Responsive | G | Pre-clinical |
| STK11 | splice_acceptor_variant | Different Mutation | Everolimus (MTOR inhibitor) | Responsive | PA | Case report |
| STK11 | splice_acceptor_variant | Complete Match | Phenformin (Anti-diabetic) | Responsive | LUAD | Pre-clinical |
| STK11 | splice_acceptor_variant | Complete Match | MTOR inhibitor | Responsive | CANCER | Pre-clinical |
| STK11 | splice_acceptor_variant | Complete Match | BET inhibitor | Resistant | L | Pre-clinical |
| STK11 | splice_acceptor_variant | Complete Match | PD1 inhibitor | Resistant | CANCER | Early trials |
| STK11 | splice_acceptor_variant | Complete Match | SRC inhibitor;PI3K/MEK inhibitor | Responsive | LUAD | Pre-clinical |
| STK11 | splice_acceptor_variant | Complete Match | MEK inhibitor | Responsive | LUAD | Pre-clinical |
| STK11 | splice_acceptor_variant | Different Alteration | MTOR inhibitor | Responsive | CANCER | Pre-clinical |
| STK11 | splice_acceptor_variant | Different Alteration | PD1 inhibitor | Resistant | CANCER | Early trials |
| STK11 | splice_acceptor_variant | Different Alteration | Phenformin (Anti-diabetic) | Responsive | LUAD | Pre-clinical |
| STK11 | splice_acceptor_variant | Different Alteration | MEK inhibitor | Responsive | LUAD | Pre-clinical |
| STK11 | splice_acceptor_variant | Different Alteration | SRC inhibitor;PI3K/MEK inhibitor | Responsive | LUAD | Pre-clinical |
| TMPRSS2 | intron_variant | Different Alteration | DNA-PKc inhibitor | Responsive | PRAD | Pre-clinical |
| TMPRSS2 | intron_variant | Different Alteration | PARP inhibitor | Responsive | PRAD | Pre-clinical |
| TMPRSS2 | Y57Y | Different Alteration | DNA-PKc inhibitor | Responsive | PRAD | Pre-clinical |
| TMPRSS2 | Y57Y | Different Alteration | PARP inhibitor | Responsive | PRAD | Pre-clinical |
| TMPRSS2 | intron_variant), TMPRSS2 MUT* (intron_variant), TMPRSS2 MUT* (intron_variant | Different Alteration | DNA-PKc inhibitor | Responsive | PRAD | Pre-clinical |
| TMPRSS2 | intron_variant), TMPRSS2 MUT* (intron_variant), TMPRSS2 MUT* (intron_variant | Different Alteration | PARP inhibitor | Responsive | PRAD | Pre-clinical |
| TMPRSS2 | intron_variant), TMPRSS2 MUT* (intron_variant | Different Alteration | DNA-PKc inhibitor | Responsive | PRAD | Pre-clinical |
| TMPRSS2 | intron_variant), TMPRSS2 MUT* (intron_variant | Different Alteration | PARP inhibitor | Responsive | PRAD | Pre-clinical |
| TP53 | R282W | Different Mutation | HSP90 inhibitor | Responsive | CANCER | Pre-clinical |
| TP53 | R282W | Complete Match | Decitabine (Chemotherapy) | Responsive | AML, MDPS | Early trials |
| TP53 | R282W | Complete Match | Abemaciclib (CDK4/6 inhibitor) | Resistant | BRCA | Early trials |
| TP53 | R282W | Different Mutation | HDM2 inhibitor | Responsive | AML | Early trials |
| TP53 | R282W | Complete Match | Cisplatin (Chemotherapy) | Resistant | FGCT, MGCT | Early trials |
| TP53 | R282W | Complete Match | AZD6738 (ATR inhibitor) | Responsive | BCL | Early trials |
| TP53 | R282W | Complete Match | Gemcitabine (Chemotherapy) | Responsive | BLCA | Pre-clinical |
| TP53 | R282W | Complete Match | Mitomycin C (Chemotherapy) | Responsive | BLCA | Pre-clinical |
| TP53 | R282W | Complete Match | WEE1 inhibitor | Responsive | HNC | Pre-clinical |
| TP53 | R282W | Complete Match | MDM2 inhibitor | Resistant | LIP | Early trials |
| TP53 | R282W | Complete Match | Doxorubicin (Anthracycline antitumor antibiotic) | Responsive | BLCA | Pre-clinical |
| TP53 | R282W | Complete Match | Pramlintide (Amylin analogue) | Responsive | THYM | Pre-clinical |
| TP53 | R282W | Complete Match | WEE1 inhibitor | Responsive | OV | Early trials |
| TP53 | R282W | Different Alteration | Pramlintide (Amylin analogue) | Responsive | THYM | Pre-clinical |
| TP53 | R282W | Different Alteration | AZD6738 (ATR inhibitor) | Responsive | BCL | Early trials |
| TP53 | splice_acceptor_variant | Different Mutation | HSP90 inhibitor | Responsive | CANCER | Pre-clinical |
| TP53 | splice_acceptor_variant | Complete Match | Decitabine (Chemotherapy) | Responsive | AML, MDPS | Early trials |
| TP53 | splice_acceptor_variant | Complete Match | Abemaciclib (CDK4/6 inhibitor) | Resistant | BRCA | Early trials |
| TP53 | splice_acceptor_variant | Different Mutation | HDM2 inhibitor | Responsive | AML | Early trials |
| TP53 | splice_acceptor_variant | Complete Match | Cisplatin (Chemotherapy) | Resistant | FGCT, MGCT | Early trials |
| TP53 | splice_acceptor_variant | Complete Match | AZD6738 (ATR inhibitor) | Responsive | BCL | Early trials |
| TP53 | splice_acceptor_variant | Complete Match | Gemcitabine (Chemotherapy) | Responsive | BLCA | Pre-clinical |
| TP53 | splice_acceptor_variant | Complete Match | Mitomycin C (Chemotherapy) | Responsive | BLCA | Pre-clinical |
| TP53 | splice_acceptor_variant | Complete Match | WEE1 inhibitor | Responsive | HNC | Pre-clinical |
| TP53 | splice_acceptor_variant | Complete Match | MDM2 inhibitor | Resistant | LIP | Early trials |
| TP53 | splice_acceptor_variant | Complete Match | Doxorubicin (Anthracycline antitumor antibiotic) | Responsive | BLCA | Pre-clinical |
| TP53 | splice_acceptor_variant | Complete Match | Pramlintide (Amylin analogue) | Responsive | THYM | Pre-clinical |
| TP53 | splice_acceptor_variant | Complete Match | WEE1 inhibitor | Responsive | OV | Early trials |
| TP53 | splice_acceptor_variant | Different Alteration | Pramlintide (Amylin analogue) | Responsive | THYM | Pre-clinical |
| TP53 | splice_acceptor_variant | Different Alteration | AZD6738 (ATR inhibitor) | Responsive | BCL | Early trials |
| TP53 | R273H | Different Mutation | HSP90 inhibitor | Responsive | CANCER | Pre-clinical |
| TP53 | R273H | Complete Match | Decitabine (Chemotherapy) | Responsive | AML, MDPS | Early trials |
| TP53 | R273H | Complete Match | Abemaciclib (CDK4/6 inhibitor) | Resistant | BRCA | Early trials |
| TP53 | R273H | Different Mutation | HDM2 inhibitor | Responsive | AML | Early trials |
| TP53 | R273H | Complete Match | Cisplatin (Chemotherapy) | Resistant | FGCT, MGCT | Early trials |
| TP53 | R273H | Complete Match | AZD6738 (ATR inhibitor) | Responsive | BCL | Early trials |
| TP53 | R273H | Complete Match | Gemcitabine (Chemotherapy) | Responsive | BLCA | Pre-clinical |
| TP53 | R273H | Complete Match | Mitomycin C (Chemotherapy) | Responsive | BLCA | Pre-clinical |
| TP53 | R273H | Complete Match | WEE1 inhibitor | Responsive | HNC | Pre-clinical |
| TP53 | R273H | Complete Match | MDM2 inhibitor | Resistant | LIP | Early trials |
| TP53 | R273H | Complete Match | Doxorubicin (Anthracycline antitumor antibiotic) | Responsive | BLCA | Pre-clinical |
| TP53 | R273H | Complete Match | Pramlintide (Amylin analogue) | Responsive | THYM | Pre-clinical |
| TP53 | R273H | Complete Match | WEE1 inhibitor | Responsive | OV | Early trials |
| TP53 | R273H | Different Alteration | Pramlintide (Amylin analogue) | Responsive | THYM | Pre-clinical |
| TP53 | R273H | Different Alteration | AZD6738 (ATR inhibitor) | Responsive | BCL | Early trials |
| TP53 | S127F | Different Mutation | HSP90 inhibitor | Responsive | CANCER | Pre-clinical |
| TP53 | S127F | Complete Match | Decitabine (Chemotherapy) | Responsive | AML, MDPS | Early trials |
| TP53 | S127F | Complete Match | Abemaciclib (CDK4/6 inhibitor) | Resistant | BRCA | Early trials |
| TP53 | S127F | Different Mutation | HDM2 inhibitor | Responsive | AML | Early trials |
| TP53 | S127F | Complete Match | Cisplatin (Chemotherapy) | Resistant | FGCT, MGCT | Early trials |
| TP53 | S127F | Complete Match | AZD6738 (ATR inhibitor) | Responsive | BCL | Early trials |
| TP53 | S127F | Complete Match | Gemcitabine (Chemotherapy) | Responsive | BLCA | Pre-clinical |
| TP53 | S127F | Complete Match | Mitomycin C (Chemotherapy) | Responsive | BLCA | Pre-clinical |
| TP53 | S127F | Complete Match | WEE1 inhibitor | Responsive | HNC | Pre-clinical |
| TP53 | S127F | Complete Match | MDM2 inhibitor | Resistant | LIP | Early trials |
| TP53 | S127F | Complete Match | Doxorubicin (Anthracycline antitumor antibiotic) | Responsive | BLCA | Pre-clinical |
| TP53 | S127F | Complete Match | Pramlintide (Amylin analogue) | Responsive | THYM | Pre-clinical |
| TP53 | S127F | Complete Match | WEE1 inhibitor | Responsive | OV | Early trials |
| TP53 | S127F | Different Alteration | Pramlintide (Amylin analogue) | Responsive | THYM | Pre-clinical |
| TP53 | S127F | Different Alteration | AZD6738 (ATR inhibitor) | Responsive | BCL | Early trials |
| TP53 | R248Q | Complete Match | HSP90 inhibitor | Responsive | CANCER | Pre-clinical |
| TP53 | R248Q | Complete Match | Decitabine (Chemotherapy) | Responsive | AML, MDPS | Early trials |
| TP53 | R248Q | Complete Match | Abemaciclib (CDK4/6 inhibitor) | Resistant | BRCA | Early trials |
| TP53 | R248Q | Different Mutation | HDM2 inhibitor | Responsive | AML | Early trials |
| TP53 | R248Q | Complete Match | Cisplatin (Chemotherapy) | Resistant | FGCT, MGCT | Early trials |
| TP53 | R248Q | Complete Match | AZD6738 (ATR inhibitor) | Responsive | BCL | Early trials |
| TP53 | R248Q | Complete Match | Gemcitabine (Chemotherapy) | Responsive | BLCA | Pre-clinical |
| TP53 | R248Q | Complete Match | Mitomycin C (Chemotherapy) | Responsive | BLCA | Pre-clinical |
| TP53 | R248Q | Complete Match | WEE1 inhibitor | Responsive | HNC | Pre-clinical |
| TP53 | R248Q | Complete Match | MDM2 inhibitor | Resistant | LIP | Early trials |
| TP53 | R248Q | Complete Match | Doxorubicin (Anthracycline antitumor antibiotic) | Responsive | BLCA | Pre-clinical |
| TP53 | R248Q | Complete Match | Pramlintide (Amylin analogue) | Responsive | THYM | Pre-clinical |
| TP53 | R248Q | Complete Match | WEE1 inhibitor | Responsive | OV | Early trials |
| TP53 | R248Q | Different Alteration | Pramlintide (Amylin analogue) | Responsive | THYM | Pre-clinical |
| TP53 | R248Q | Different Alteration | AZD6738 (ATR inhibitor) | Responsive | BCL | Early trials |
| TP53 | R213L | Different Mutation | HSP90 inhibitor | Responsive | CANCER | Pre-clinical |
| TP53 | R213L | Complete Match | Decitabine (Chemotherapy) | Responsive | AML, MDPS | Early trials |
| TP53 | R213L | Complete Match | Abemaciclib (CDK4/6 inhibitor) | Resistant | BRCA | Early trials |
| TP53 | R213L | Different Mutation | HDM2 inhibitor | Responsive | AML | Early trials |
| TP53 | R213L | Complete Match | Cisplatin (Chemotherapy) | Resistant | FGCT, MGCT | Early trials |
| TP53 | R213L | Complete Match | AZD6738 (ATR inhibitor) | Responsive | BCL | Early trials |
| TP53 | R213L | Complete Match | Gemcitabine (Chemotherapy) | Responsive | BLCA | Pre-clinical |
| TP53 | R213L | Complete Match | Mitomycin C (Chemotherapy) | Responsive | BLCA | Pre-clinical |
| TP53 | R213L | Complete Match | WEE1 inhibitor | Responsive | HNC | Pre-clinical |
| TP53 | R213L | Complete Match | MDM2 inhibitor | Resistant | LIP | Early trials |
| TP53 | R213L | Complete Match | Doxorubicin (Anthracycline antitumor antibiotic) | Responsive | BLCA | Pre-clinical |
| TP53 | R213L | Complete Match | Pramlintide (Amylin analogue) | Responsive | THYM | Pre-clinical |
| TP53 | R213L | Complete Match | WEE1 inhibitor | Responsive | OV | Early trials |
| TP53 | R213L | Different Alteration | Pramlintide (Amylin analogue) | Responsive | THYM | Pre-clinical |
| TP53 | R213L | Different Alteration | AZD6738 (ATR inhibitor) | Responsive | BCL | Early trials |
| TP53 | V272L | Different Mutation | HSP90 inhibitor | Responsive | CANCER | Pre-clinical |
| TP53 | V272L | Complete Match | Decitabine (Chemotherapy) | Responsive | AML, MDPS | Early trials |
| TP53 | V272L | Complete Match | Abemaciclib (CDK4/6 inhibitor) | Resistant | BRCA | Early trials |
| TP53 | V272L | Different Mutation | HDM2 inhibitor | Responsive | AML | Early trials |
| TP53 | V272L | Complete Match | Cisplatin (Chemotherapy) | Resistant | FGCT, MGCT | Early trials |
| TP53 | V272L | Complete Match | AZD6738 (ATR inhibitor) | Responsive | BCL | Early trials |
| TP53 | V272L | Complete Match | Gemcitabine (Chemotherapy) | Responsive | BLCA | Pre-clinical |
| TP53 | V272L | Complete Match | Mitomycin C (Chemotherapy) | Responsive | BLCA | Pre-clinical |
| TP53 | V272L | Complete Match | WEE1 inhibitor | Responsive | HNC | Pre-clinical |
| TP53 | V272L | Complete Match | MDM2 inhibitor | Resistant | LIP | Early trials |
| TP53 | V272L | Complete Match | Doxorubicin (Anthracycline antitumor antibiotic) | Responsive | BLCA | Pre-clinical |
| TP53 | V272L | Complete Match | Pramlintide (Amylin analogue) | Responsive | THYM | Pre-clinical |
| TP53 | V272L | Complete Match | WEE1 inhibitor | Responsive | OV | Early trials |
| TP53 | V272L | Different Alteration | Pramlintide (Amylin analogue) | Responsive | THYM | Pre-clinical |
| TP53 | V272L | Different Alteration | AZD6738 (ATR inhibitor) | Responsive | BCL | Early trials |
| TP53 | Y163C | Different Mutation | HSP90 inhibitor | Responsive | CANCER | Pre-clinical |
| TP53 | Y163C | Complete Match | Decitabine (Chemotherapy) | Responsive | AML, MDPS | Early trials |
| TP53 | Y163C | Complete Match | Abemaciclib (CDK4/6 inhibitor) | Resistant | BRCA | Early trials |
| TP53 | Y163C | Different Mutation | HDM2 inhibitor | Responsive | AML | Early trials |
| TP53 | Y163C | Complete Match | Cisplatin (Chemotherapy) | Resistant | FGCT, MGCT | Early trials |
| TP53 | Y163C | Complete Match | AZD6738 (ATR inhibitor) | Responsive | BCL | Early trials |
| TP53 | Y163C | Complete Match | Gemcitabine (Chemotherapy) | Responsive | BLCA | Pre-clinical |
| TP53 | Y163C | Complete Match | Mitomycin C (Chemotherapy) | Responsive | BLCA | Pre-clinical |
| TP53 | Y163C | Complete Match | WEE1 inhibitor | Responsive | HNC | Pre-clinical |
| TP53 | Y163C | Complete Match | MDM2 inhibitor | Resistant | LIP | Early trials |
| TP53 | Y163C | Complete Match | Doxorubicin (Anthracycline antitumor antibiotic) | Responsive | BLCA | Pre-clinical |
| TP53 | Y163C | Complete Match | Pramlintide (Amylin analogue) | Responsive | THYM | Pre-clinical |
| TP53 | Y163C | Complete Match | WEE1 inhibitor | Responsive | OV | Early trials |
| TP53 | Y163C | Different Alteration | Pramlintide (Amylin analogue) | Responsive | THYM | Pre-clinical |
| TP53 | Y163C | Different Alteration | AZD6738 (ATR inhibitor) | Responsive | BCL | Early trials |
| TP53 | G266V | Different Mutation | HSP90 inhibitor | Responsive | CANCER | Pre-clinical |
| TP53 | G266V | Complete Match | Decitabine (Chemotherapy) | Responsive | AML, MDPS | Early trials |
| TP53 | G266V | Complete Match | Abemaciclib (CDK4/6 inhibitor) | Resistant | BRCA | Early trials |
| TP53 | G266V | Different Mutation | HDM2 inhibitor | Responsive | AML | Early trials |
| TP53 | G266V | Complete Match | Cisplatin (Chemotherapy) | Resistant | FGCT, MGCT | Early trials |
| TP53 | G266V | Complete Match | AZD6738 (ATR inhibitor) | Responsive | BCL | Early trials |
| TP53 | G266V | Complete Match | Gemcitabine (Chemotherapy) | Responsive | BLCA | Pre-clinical |
| TP53 | G266V | Complete Match | Mitomycin C (Chemotherapy) | Responsive | BLCA | Pre-clinical |
| TP53 | G266V | Complete Match | WEE1 inhibitor | Responsive | HNC | Pre-clinical |
| TP53 | G266V | Complete Match | MDM2 inhibitor | Resistant | LIP | Early trials |
| TP53 | G266V | Complete Match | Doxorubicin (Anthracycline antitumor antibiotic) | Responsive | BLCA | Pre-clinical |
| TP53 | G266V | Complete Match | Pramlintide (Amylin analogue) | Responsive | THYM | Pre-clinical |
| TP53 | G266V | Complete Match | WEE1 inhibitor | Responsive | OV | Early trials |
| TP53 | G266V | Different Alteration | Pramlintide (Amylin analogue) | Responsive | THYM | Pre-clinical |
| TP53 | G266V | Different Alteration | AZD6738 (ATR inhibitor) | Responsive | BCL | Early trials |
| TP53 | S127Y | Different Mutation | HSP90 inhibitor | Responsive | CANCER | Pre-clinical |
| TP53 | S127Y | Complete Match | Decitabine (Chemotherapy) | Responsive | AML, MDPS | Early trials |
| TP53 | S127Y | Complete Match | Abemaciclib (CDK4/6 inhibitor) | Resistant | BRCA | Early trials |
| TP53 | S127Y | Different Mutation | HDM2 inhibitor | Responsive | AML | Early trials |
| TP53 | S127Y | Complete Match | Cisplatin (Chemotherapy) | Resistant | FGCT, MGCT | Early trials |
| TP53 | S127Y | Complete Match | AZD6738 (ATR inhibitor) | Responsive | BCL | Early trials |
| TP53 | S127Y | Complete Match | Gemcitabine (Chemotherapy) | Responsive | BLCA | Pre-clinical |
| TP53 | S127Y | Complete Match | Mitomycin C (Chemotherapy) | Responsive | BLCA | Pre-clinical |
| TP53 | S127Y | Complete Match | WEE1 inhibitor | Responsive | HNC | Pre-clinical |
| TP53 | S127Y | Complete Match | MDM2 inhibitor | Resistant | LIP | Early trials |
| TP53 | S127Y | Complete Match | Doxorubicin (Anthracycline antitumor antibiotic) | Responsive | BLCA | Pre-clinical |
| TP53 | S127Y | Complete Match | Pramlintide (Amylin analogue) | Responsive | THYM | Pre-clinical |
| TP53 | S127Y | Complete Match | WEE1 inhibitor | Responsive | OV | Early trials |
| TP53 | S127Y | Different Alteration | Pramlintide (Amylin analogue) | Responsive | THYM | Pre-clinical |
| TP53 | S127Y | Different Alteration | AZD6738 (ATR inhibitor) | Responsive | BCL | Early trials |
| TP53 | I254S | Different Mutation | HSP90 inhibitor | Responsive | CANCER | Pre-clinical |
| TP53 | I254S | Complete Match | Decitabine (Chemotherapy) | Responsive | AML, MDPS | Early trials |
| TP53 | I254S | Complete Match | Abemaciclib (CDK4/6 inhibitor) | Resistant | BRCA | Early trials |
| TP53 | I254S | Different Mutation | HDM2 inhibitor | Responsive | AML | Early trials |
| TP53 | I254S | Complete Match | Cisplatin (Chemotherapy) | Resistant | FGCT, MGCT | Early trials |
| TP53 | I254S | Complete Match | AZD6738 (ATR inhibitor) | Responsive | BCL | Early trials |
| TP53 | I254S | Complete Match | Gemcitabine (Chemotherapy) | Responsive | BLCA | Pre-clinical |
| TP53 | I254S | Complete Match | Mitomycin C (Chemotherapy) | Responsive | BLCA | Pre-clinical |
| TP53 | I254S | Complete Match | WEE1 inhibitor | Responsive | HNC | Pre-clinical |
| TP53 | I254S | Complete Match | MDM2 inhibitor | Resistant | LIP | Early trials |
| TP53 | I254S | Complete Match | Doxorubicin (Anthracycline antitumor antibiotic) | Responsive | BLCA | Pre-clinical |
| TP53 | I254S | Complete Match | Pramlintide (Amylin analogue) | Responsive | THYM | Pre-clinical |
| TP53 | I254S | Complete Match | WEE1 inhibitor | Responsive | OV | Early trials |
| TP53 | I254S | Different Alteration | Pramlintide (Amylin analogue) | Responsive | THYM | Pre-clinical |
| TP53 | I254S | Different Alteration | AZD6738 (ATR inhibitor) | Responsive | BCL | Early trials |
| TP53 | R248W | Different Mutation | HSP90 inhibitor | Responsive | CANCER | Pre-clinical |
| TP53 | R248W | Complete Match | Decitabine (Chemotherapy) | Responsive | AML, MDPS | Early trials |
| TP53 | R248W | Complete Match | Abemaciclib (CDK4/6 inhibitor) | Resistant | BRCA | Early trials |
| TP53 | R248W | Different Mutation | HDM2 inhibitor | Responsive | AML | Early trials |
| TP53 | R248W | Complete Match | Cisplatin (Chemotherapy) | Resistant | FGCT, MGCT | Early trials |
| TP53 | R248W | Complete Match | AZD6738 (ATR inhibitor) | Responsive | BCL | Early trials |
| TP53 | R248W | Complete Match | Gemcitabine (Chemotherapy) | Responsive | BLCA | Pre-clinical |
| TP53 | R248W | Complete Match | Mitomycin C (Chemotherapy) | Responsive | BLCA | Pre-clinical |
| TP53 | R248W | Complete Match | WEE1 inhibitor | Responsive | HNC | Pre-clinical |
| TP53 | R248W | Complete Match | MDM2 inhibitor | Resistant | LIP | Early trials |
| TP53 | R248W | Complete Match | Doxorubicin (Anthracycline antitumor antibiotic) | Responsive | BLCA | Pre-clinical |
| TP53 | R248W | Complete Match | Pramlintide (Amylin analogue) | Responsive | THYM | Pre-clinical |
| TP53 | R248W | Complete Match | WEE1 inhibitor | Responsive | OV | Early trials |
| TP53 | R248W | Different Alteration | Pramlintide (Amylin analogue) | Responsive | THYM | Pre-clinical |
| TP53 | R248W | Different Alteration | AZD6738 (ATR inhibitor) | Responsive | BCL | Early trials |
| TP53 | W53* | Different Mutation | HSP90 inhibitor | Responsive | CANCER | Pre-clinical |
| TP53 | W53* | Complete Match | Decitabine (Chemotherapy) | Responsive | AML, MDPS | Early trials |
| TP53 | W53* | Complete Match | Abemaciclib (CDK4/6 inhibitor) | Resistant | BRCA | Early trials |
| TP53 | W53* | Different Mutation | HDM2 inhibitor | Responsive | AML | Early trials |
| TP53 | W53* | Complete Match | Cisplatin (Chemotherapy) | Resistant | FGCT, MGCT | Early trials |
| TP53 | W53* | Complete Match | AZD6738 (ATR inhibitor) | Responsive | BCL | Early trials |
| TP53 | W53* | Complete Match | Gemcitabine (Chemotherapy) | Responsive | BLCA | Pre-clinical |
| TP53 | W53* | Complete Match | Mitomycin C (Chemotherapy) | Responsive | BLCA | Pre-clinical |
| TP53 | W53* | Complete Match | WEE1 inhibitor | Responsive | HNC | Pre-clinical |
| TP53 | W53* | Complete Match | MDM2 inhibitor | Resistant | LIP | Early trials |
| TP53 | W53* | Complete Match | Doxorubicin (Anthracycline antitumor antibiotic) | Responsive | BLCA | Pre-clinical |
| TP53 | W53* | Complete Match | Pramlintide (Amylin analogue) | Responsive | THYM | Pre-clinical |
| TP53 | W53* | Complete Match | WEE1 inhibitor | Responsive | OV | Early trials |
| TP53 | W53* | Different Alteration | Pramlintide (Amylin analogue) | Responsive | THYM | Pre-clinical |
| TP53 | W53* | Different Alteration | AZD6738 (ATR inhibitor) | Responsive | BCL | Early trials |
| TP53 | Y126S | Different Mutation | HSP90 inhibitor | Responsive | CANCER | Pre-clinical |
| TP53 | Y126S | Complete Match | Decitabine (Chemotherapy) | Responsive | AML, MDPS | Early trials |
| TP53 | Y126S | Complete Match | Abemaciclib (CDK4/6 inhibitor) | Resistant | BRCA | Early trials |
| TP53 | Y126S | Different Mutation | HDM2 inhibitor | Responsive | AML | Early trials |
| TP53 | Y126S | Complete Match | Cisplatin (Chemotherapy) | Resistant | FGCT, MGCT | Early trials |
| TP53 | Y126S | Complete Match | AZD6738 (ATR inhibitor) | Responsive | BCL | Early trials |
| TP53 | Y126S | Complete Match | Gemcitabine (Chemotherapy) | Responsive | BLCA | Pre-clinical |
| TP53 | Y126S | Complete Match | Mitomycin C (Chemotherapy) | Responsive | BLCA | Pre-clinical |
| TP53 | Y126S | Complete Match | WEE1 inhibitor | Responsive | HNC | Pre-clinical |
| TP53 | Y126S | Complete Match | MDM2 inhibitor | Resistant | LIP | Early trials |
| TP53 | Y126S | Complete Match | Doxorubicin (Anthracycline antitumor antibiotic) | Responsive | BLCA | Pre-clinical |
| TP53 | Y126S | Complete Match | Pramlintide (Amylin analogue) | Responsive | THYM | Pre-clinical |
| TP53 | Y126S | Complete Match | WEE1 inhibitor | Responsive | OV | Early trials |
| TP53 | Y126S | Different Alteration | Pramlintide (Amylin analogue) | Responsive | THYM | Pre-clinical |
| TP53 | Y126S | Different Alteration | AZD6738 (ATR inhibitor) | Responsive | BCL | Early trials |
| TP53 | R273C | Different Mutation | HSP90 inhibitor | Responsive | CANCER | Pre-clinical |
| TP53 | R273C | Complete Match | Decitabine (Chemotherapy) | Responsive | AML, MDPS | Early trials |
| TP53 | R273C | Complete Match | Abemaciclib (CDK4/6 inhibitor) | Resistant | BRCA | Early trials |
| TP53 | R273C | Different Mutation | HDM2 inhibitor | Responsive | AML | Early trials |
| TP53 | R273C | Complete Match | Cisplatin (Chemotherapy) | Resistant | FGCT, MGCT | Early trials |
| TP53 | R273C | Complete Match | AZD6738 (ATR inhibitor) | Responsive | BCL | Early trials |
| TP53 | R273C | Complete Match | Gemcitabine (Chemotherapy) | Responsive | BLCA | Pre-clinical |
| TP53 | R273C | Complete Match | Mitomycin C (Chemotherapy) | Responsive | BLCA | Pre-clinical |
| TP53 | R273C | Complete Match | WEE1 inhibitor | Responsive | HNC | Pre-clinical |
| TP53 | R273C | Complete Match | MDM2 inhibitor | Resistant | LIP | Early trials |
| TP53 | R273C | Complete Match | Doxorubicin (Anthracycline antitumor antibiotic) | Responsive | BLCA | Pre-clinical |
| TP53 | R273C | Complete Match | Pramlintide (Amylin analogue) | Responsive | THYM | Pre-clinical |
| TP53 | R273C | Complete Match | WEE1 inhibitor | Responsive | OV | Early trials |
| TP53 | R273C | Different Alteration | Pramlintide (Amylin analogue) | Responsive | THYM | Pre-clinical |
| TP53 | R273C | Different Alteration | AZD6738 (ATR inhibitor) | Responsive | BCL | Early trials |
| TP53 | R196P | Different Mutation | HSP90 inhibitor | Responsive | CANCER | Pre-clinical |
| TP53 | R196P | Complete Match | Decitabine (Chemotherapy) | Responsive | AML, MDPS | Early trials |
| TP53 | R196P | Complete Match | Abemaciclib (CDK4/6 inhibitor) | Resistant | BRCA | Early trials |
| TP53 | R196P | Different Mutation | HDM2 inhibitor | Responsive | AML | Early trials |
| TP53 | R196P | Complete Match | Cisplatin (Chemotherapy) | Resistant | FGCT, MGCT | Early trials |
| TP53 | R196P | Complete Match | AZD6738 (ATR inhibitor) | Responsive | BCL | Early trials |
| TP53 | R196P | Complete Match | Gemcitabine (Chemotherapy) | Responsive | BLCA | Pre-clinical |
| TP53 | R196P | Complete Match | Mitomycin C (Chemotherapy) | Responsive | BLCA | Pre-clinical |
| TP53 | R196P | Complete Match | WEE1 inhibitor | Responsive | HNC | Pre-clinical |
| TP53 | R196P | Complete Match | MDM2 inhibitor | Resistant | LIP | Early trials |
| TP53 | R196P | Complete Match | Doxorubicin (Anthracycline antitumor antibiotic) | Responsive | BLCA | Pre-clinical |
| TP53 | R196P | Complete Match | Pramlintide (Amylin analogue) | Responsive | THYM | Pre-clinical |
| TP53 | R196P | Complete Match | WEE1 inhibitor | Responsive | OV | Early trials |
| TP53 | R196P | Different Alteration | Pramlintide (Amylin analogue) | Responsive | THYM | Pre-clinical |
| TP53 | R196P | Different Alteration | AZD6738 (ATR inhibitor) | Responsive | BCL | Early trials |
| TP53 | R282G | Different Mutation | HSP90 inhibitor | Responsive | CANCER | Pre-clinical |
| TP53 | R282G | Complete Match | Decitabine (Chemotherapy) | Responsive | AML, MDPS | Early trials |
| TP53 | R282G | Complete Match | Abemaciclib (CDK4/6 inhibitor) | Resistant | BRCA | Early trials |
| TP53 | R282G | Different Mutation | HDM2 inhibitor | Responsive | AML | Early trials |
| TP53 | R282G | Complete Match | Cisplatin (Chemotherapy) | Resistant | FGCT, MGCT | Early trials |
| TP53 | R282G | Complete Match | AZD6738 (ATR inhibitor) | Responsive | BCL | Early trials |
| TP53 | R282G | Complete Match | Gemcitabine (Chemotherapy) | Responsive | BLCA | Pre-clinical |
| TP53 | R282G | Complete Match | Mitomycin C (Chemotherapy) | Responsive | BLCA | Pre-clinical |
| TP53 | R282G | Complete Match | WEE1 inhibitor | Responsive | HNC | Pre-clinical |
| TP53 | R282G | Complete Match | MDM2 inhibitor | Resistant | LIP | Early trials |
| TP53 | R282G | Complete Match | Doxorubicin (Anthracycline antitumor antibiotic) | Responsive | BLCA | Pre-clinical |
| TP53 | R282G | Complete Match | Pramlintide (Amylin analogue) | Responsive | THYM | Pre-clinical |
| TP53 | R282G | Complete Match | WEE1 inhibitor | Responsive | OV | Early trials |
| TP53 | R282G | Different Alteration | Pramlintide (Amylin analogue) | Responsive | THYM | Pre-clinical |
| TP53 | R282G | Different Alteration | AZD6738 (ATR inhibitor) | Responsive | BCL | Early trials |
| TP53 | Y205S | Different Mutation | HSP90 inhibitor | Responsive | CANCER | Pre-clinical |
| TP53 | Y205S | Complete Match | Decitabine (Chemotherapy) | Responsive | AML, MDPS | Early trials |
| TP53 | Y205S | Complete Match | Abemaciclib (CDK4/6 inhibitor) | Resistant | BRCA | Early trials |
| TP53 | Y205S | Different Mutation | HDM2 inhibitor | Responsive | AML | Early trials |
| TP53 | Y205S | Complete Match | Cisplatin (Chemotherapy) | Resistant | FGCT, MGCT | Early trials |
| TP53 | Y205S | Complete Match | AZD6738 (ATR inhibitor) | Responsive | BCL | Early trials |
| TP53 | Y205S | Complete Match | Gemcitabine (Chemotherapy) | Responsive | BLCA | Pre-clinical |
| TP53 | Y205S | Complete Match | Mitomycin C (Chemotherapy) | Responsive | BLCA | Pre-clinical |
| TP53 | Y205S | Complete Match | WEE1 inhibitor | Responsive | HNC | Pre-clinical |
| TP53 | Y205S | Complete Match | MDM2 inhibitor | Resistant | LIP | Early trials |
| TP53 | Y205S | Complete Match | Doxorubicin (Anthracycline antitumor antibiotic) | Responsive | BLCA | Pre-clinical |
| TP53 | Y205S | Complete Match | Pramlintide (Amylin analogue) | Responsive | THYM | Pre-clinical |
| TP53 | Y205S | Complete Match | WEE1 inhibitor | Responsive | OV | Early trials |
| TP53 | Y205S | Different Alteration | Pramlintide (Amylin analogue) | Responsive | THYM | Pre-clinical |
| TP53 | Y205S | Different Alteration | AZD6738 (ATR inhibitor) | Responsive | BCL | Early trials |
| TP53 | R175H | Complete Match | HSP90 inhibitor | Responsive | CANCER | Pre-clinical |
| TP53 | R175H | Complete Match | Decitabine (Chemotherapy) | Responsive | AML, MDPS | Early trials |
| TP53 | R175H | Complete Match | Abemaciclib (CDK4/6 inhibitor) | Resistant | BRCA | Early trials |
| TP53 | R175H | Different Mutation | HDM2 inhibitor | Responsive | AML | Early trials |
| TP53 | R175H | Complete Match | Cisplatin (Chemotherapy) | Resistant | FGCT, MGCT | Early trials |
| TP53 | R175H | Complete Match | AZD6738 (ATR inhibitor) | Responsive | BCL | Early trials |
| TP53 | R175H | Complete Match | Gemcitabine (Chemotherapy) | Responsive | BLCA | Pre-clinical |
| TP53 | R175H | Complete Match | Mitomycin C (Chemotherapy) | Responsive | BLCA | Pre-clinical |
| TP53 | R175H | Complete Match | WEE1 inhibitor | Responsive | HNC | Pre-clinical |
| TP53 | R175H | Complete Match | MDM2 inhibitor | Resistant | LIP | Early trials |
| TP53 | R175H | Complete Match | Doxorubicin (Anthracycline antitumor antibiotic) | Responsive | BLCA | Pre-clinical |
| TP53 | R175H | Complete Match | Pramlintide (Amylin analogue) | Responsive | THYM | Pre-clinical |
| TP53 | R175H | Complete Match | WEE1 inhibitor | Responsive | OV | Early trials |
| TP53 | R175H | Different Alteration | Pramlintide (Amylin analogue) | Responsive | THYM | Pre-clinical |
| TP53 | R175H | Different Alteration | AZD6738 (ATR inhibitor) | Responsive | BCL | Early trials |
| TP53 | H193Y | Different Mutation | HSP90 inhibitor | Responsive | CANCER | Pre-clinical |
| TP53 | H193Y | Complete Match | Decitabine (Chemotherapy) | Responsive | AML, MDPS | Early trials |
| TP53 | H193Y | Complete Match | Abemaciclib (CDK4/6 inhibitor) | Resistant | BRCA | Early trials |
| TP53 | H193Y | Different Mutation | HDM2 inhibitor | Responsive | AML | Early trials |
| TP53 | H193Y | Complete Match | Cisplatin (Chemotherapy) | Resistant | FGCT, MGCT | Early trials |
| TP53 | H193Y | Complete Match | AZD6738 (ATR inhibitor) | Responsive | BCL | Early trials |
| TP53 | H193Y | Complete Match | Gemcitabine (Chemotherapy) | Responsive | BLCA | Pre-clinical |
| TP53 | H193Y | Complete Match | Mitomycin C (Chemotherapy) | Responsive | BLCA | Pre-clinical |
| TP53 | H193Y | Complete Match | WEE1 inhibitor | Responsive | HNC | Pre-clinical |
| TP53 | H193Y | Complete Match | MDM2 inhibitor | Resistant | LIP | Early trials |
| TP53 | H193Y | Complete Match | Doxorubicin (Anthracycline antitumor antibiotic) | Responsive | BLCA | Pre-clinical |
| TP53 | H193Y | Complete Match | Pramlintide (Amylin analogue) | Responsive | THYM | Pre-clinical |
| TP53 | H193Y | Complete Match | WEE1 inhibitor | Responsive | OV | Early trials |
| TP53 | H193Y | Different Alteration | Pramlintide (Amylin analogue) | Responsive | THYM | Pre-clinical |
| TP53 | H193Y | Different Alteration | AZD6738 (ATR inhibitor) | Responsive | BCL | Early trials |
| TP53 | G245S | Different Mutation | HSP90 inhibitor | Responsive | CANCER | Pre-clinical |
| TP53 | G245S | Complete Match | Decitabine (Chemotherapy) | Responsive | AML, MDPS | Early trials |
| TP53 | G245S | Complete Match | Abemaciclib (CDK4/6 inhibitor) | Resistant | BRCA | Early trials |
| TP53 | G245S | Different Mutation | HDM2 inhibitor | Responsive | AML | Early trials |
| TP53 | G245S | Complete Match | Cisplatin (Chemotherapy) | Resistant | FGCT, MGCT | Early trials |
| TP53 | G245S | Complete Match | AZD6738 (ATR inhibitor) | Responsive | BCL | Early trials |
| TP53 | G245S | Complete Match | Gemcitabine (Chemotherapy) | Responsive | BLCA | Pre-clinical |
| TP53 | G245S | Complete Match | Mitomycin C (Chemotherapy) | Responsive | BLCA | Pre-clinical |
| TP53 | G245S | Complete Match | WEE1 inhibitor | Responsive | HNC | Pre-clinical |
| TP53 | G245S | Complete Match | MDM2 inhibitor | Resistant | LIP | Early trials |
| TP53 | G245S | Complete Match | Doxorubicin (Anthracycline antitumor antibiotic) | Responsive | BLCA | Pre-clinical |
| TP53 | G245S | Complete Match | Pramlintide (Amylin analogue) | Responsive | THYM | Pre-clinical |
| TP53 | G245S | Complete Match | WEE1 inhibitor | Responsive | OV | Early trials |
| TP53 | G245S | Different Alteration | Pramlintide (Amylin analogue) | Responsive | THYM | Pre-clinical |
| TP53 | G245S | Different Alteration | AZD6738 (ATR inhibitor) | Responsive | BCL | Early trials |
| TP53 | S215G | Different Mutation | HSP90 inhibitor | Responsive | CANCER | Pre-clinical |
| TP53 | S215G | Complete Match | Decitabine (Chemotherapy) | Responsive | AML, MDPS | Early trials |
| TP53 | S215G | Complete Match | Abemaciclib (CDK4/6 inhibitor) | Resistant | BRCA | Early trials |
| TP53 | S215G | Different Mutation | HDM2 inhibitor | Responsive | AML | Early trials |
| TP53 | S215G | Complete Match | Cisplatin (Chemotherapy) | Resistant | FGCT, MGCT | Early trials |
| TP53 | S215G | Complete Match | AZD6738 (ATR inhibitor) | Responsive | BCL | Early trials |
| TP53 | S215G | Complete Match | Gemcitabine (Chemotherapy) | Responsive | BLCA | Pre-clinical |
| TP53 | S215G | Complete Match | Mitomycin C (Chemotherapy) | Responsive | BLCA | Pre-clinical |
| TP53 | S215G | Complete Match | WEE1 inhibitor | Responsive | HNC | Pre-clinical |
| TP53 | S215G | Complete Match | MDM2 inhibitor | Resistant | LIP | Early trials |
| TP53 | S215G | Complete Match | Doxorubicin (Anthracycline antitumor antibiotic) | Responsive | BLCA | Pre-clinical |
| TP53 | S215G | Complete Match | Pramlintide (Amylin analogue) | Responsive | THYM | Pre-clinical |
| TP53 | S215G | Complete Match | WEE1 inhibitor | Responsive | OV | Early trials |
| TP53 | S215G | Different Alteration | Pramlintide (Amylin analogue) | Responsive | THYM | Pre-clinical |
| TP53 | S215G | Different Alteration | AZD6738 (ATR inhibitor) | Responsive | BCL | Early trials |
| TP53 | Y327* | Different Mutation | HSP90 inhibitor | Responsive | CANCER | Pre-clinical |
| TP53 | Y327* | Complete Match | Decitabine (Chemotherapy) | Responsive | AML, MDPS | Early trials |
| TP53 | Y327* | Complete Match | Abemaciclib (CDK4/6 inhibitor) | Resistant | BRCA | Early trials |
| TP53 | Y327* | Different Mutation | HDM2 inhibitor | Responsive | AML | Early trials |
| TP53 | Y327* | Complete Match | Cisplatin (Chemotherapy) | Resistant | FGCT, MGCT | Early trials |
| TP53 | Y327* | Complete Match | AZD6738 (ATR inhibitor) | Responsive | BCL | Early trials |
| TP53 | Y327* | Complete Match | Gemcitabine (Chemotherapy) | Responsive | BLCA | Pre-clinical |
| TP53 | Y327* | Complete Match | Mitomycin C (Chemotherapy) | Responsive | BLCA | Pre-clinical |
| TP53 | Y327* | Complete Match | WEE1 inhibitor | Responsive | HNC | Pre-clinical |
| TP53 | Y327* | Complete Match | MDM2 inhibitor | Resistant | LIP | Early trials |
| TP53 | Y327* | Complete Match | Doxorubicin (Anthracycline antitumor antibiotic) | Responsive | BLCA | Pre-clinical |
| TP53 | Y327* | Complete Match | Pramlintide (Amylin analogue) | Responsive | THYM | Pre-clinical |
| TP53 | Y327* | Complete Match | WEE1 inhibitor | Responsive | OV | Early trials |
| TP53 | Y327* | Different Alteration | Pramlintide (Amylin analogue) | Responsive | THYM | Pre-clinical |
| TP53 | Y327* | Different Alteration | AZD6738 (ATR inhibitor) | Responsive | BCL | Early trials |
| TP53 | K132R | Different Mutation | HSP90 inhibitor | Responsive | CANCER | Pre-clinical |
| TP53 | K132R | Complete Match | Decitabine (Chemotherapy) | Responsive | AML, MDPS | Early trials |
| TP53 | K132R | Complete Match | Abemaciclib (CDK4/6 inhibitor) | Resistant | BRCA | Early trials |
| TP53 | K132R | Different Mutation | HDM2 inhibitor | Responsive | AML | Early trials |
| TP53 | K132R | Complete Match | Cisplatin (Chemotherapy) | Resistant | FGCT, MGCT | Early trials |
| TP53 | K132R | Complete Match | AZD6738 (ATR inhibitor) | Responsive | BCL | Early trials |
| TP53 | K132R | Complete Match | Gemcitabine (Chemotherapy) | Responsive | BLCA | Pre-clinical |
| TP53 | K132R | Complete Match | Mitomycin C (Chemotherapy) | Responsive | BLCA | Pre-clinical |
| TP53 | K132R | Complete Match | WEE1 inhibitor | Responsive | HNC | Pre-clinical |
| TP53 | K132R | Complete Match | MDM2 inhibitor | Resistant | LIP | Early trials |
| TP53 | K132R | Complete Match | Doxorubicin (Anthracycline antitumor antibiotic) | Responsive | BLCA | Pre-clinical |
| TP53 | K132R | Complete Match | Pramlintide (Amylin analogue) | Responsive | THYM | Pre-clinical |
| TP53 | K132R | Complete Match | WEE1 inhibitor | Responsive | OV | Early trials |
| TP53 | K132R | Different Alteration | Pramlintide (Amylin analogue) | Responsive | THYM | Pre-clinical |
| TP53 | K132R | Different Alteration | AZD6738 (ATR inhibitor) | Responsive | BCL | Early trials |
| TP53 | R196* | Different Mutation | HSP90 inhibitor | Responsive | CANCER | Pre-clinical |
| TP53 | R196* | Complete Match | Decitabine (Chemotherapy) | Responsive | AML, MDPS | Early trials |
| TP53 | R196* | Complete Match | Abemaciclib (CDK4/6 inhibitor) | Resistant | BRCA | Early trials |
| TP53 | R196* | Different Mutation | HDM2 inhibitor | Responsive | AML | Early trials |
| TP53 | R196* | Complete Match | Cisplatin (Chemotherapy) | Resistant | FGCT, MGCT | Early trials |
| TP53 | R196* | Complete Match | AZD6738 (ATR inhibitor) | Responsive | BCL | Early trials |
| TP53 | R196* | Complete Match | Gemcitabine (Chemotherapy) | Responsive | BLCA | Pre-clinical |
| TP53 | R196* | Complete Match | Mitomycin C (Chemotherapy) | Responsive | BLCA | Pre-clinical |
| TP53 | R196* | Complete Match | WEE1 inhibitor | Responsive | HNC | Pre-clinical |
| TP53 | R196* | Complete Match | MDM2 inhibitor | Resistant | LIP | Early trials |
| TP53 | R196* | Complete Match | Doxorubicin (Anthracycline antitumor antibiotic) | Responsive | BLCA | Pre-clinical |
| TP53 | R196* | Complete Match | Pramlintide (Amylin analogue) | Responsive | THYM | Pre-clinical |
| TP53 | R196* | Complete Match | WEE1 inhibitor | Responsive | OV | Early trials |
| TP53 | R196* | Different Alteration | Pramlintide (Amylin analogue) | Responsive | THYM | Pre-clinical |
| TP53 | R196* | Different Alteration | AZD6738 (ATR inhibitor) | Responsive | BCL | Early trials |
| TP53 | G266R | Different Mutation | HSP90 inhibitor | Responsive | CANCER | Pre-clinical |
| TP53 | G266R | Complete Match | Decitabine (Chemotherapy) | Responsive | AML, MDPS | Early trials |
| TP53 | G266R | Complete Match | Abemaciclib (CDK4/6 inhibitor) | Resistant | BRCA | Early trials |
| TP53 | G266R | Different Mutation | HDM2 inhibitor | Responsive | AML | Early trials |
| TP53 | G266R | Complete Match | Cisplatin (Chemotherapy) | Resistant | FGCT, MGCT | Early trials |
| TP53 | G266R | Complete Match | AZD6738 (ATR inhibitor) | Responsive | BCL | Early trials |
| TP53 | G266R | Complete Match | Gemcitabine (Chemotherapy) | Responsive | BLCA | Pre-clinical |
| TP53 | G266R | Complete Match | Mitomycin C (Chemotherapy) | Responsive | BLCA | Pre-clinical |
| TP53 | G266R | Complete Match | WEE1 inhibitor | Responsive | HNC | Pre-clinical |
| TP53 | G266R | Complete Match | MDM2 inhibitor | Resistant | LIP | Early trials |
| TP53 | G266R | Complete Match | Doxorubicin (Anthracycline antitumor antibiotic) | Responsive | BLCA | Pre-clinical |
| TP53 | G266R | Complete Match | Pramlintide (Amylin analogue) | Responsive | THYM | Pre-clinical |
| TP53 | G266R | Complete Match | WEE1 inhibitor | Responsive | OV | Early trials |
| TP53 | G266R | Different Alteration | Pramlintide (Amylin analogue) | Responsive | THYM | Pre-clinical |
| TP53 | G266R | Different Alteration | AZD6738 (ATR inhibitor) | Responsive | BCL | Early trials |
| TP53 | A159V | Different Mutation | HSP90 inhibitor | Responsive | CANCER | Pre-clinical |
| TP53 | A159V | Complete Match | Decitabine (Chemotherapy) | Responsive | AML, MDPS | Early trials |
| TP53 | A159V | Complete Match | Abemaciclib (CDK4/6 inhibitor) | Resistant | BRCA | Early trials |
| TP53 | A159V | Different Mutation | HDM2 inhibitor | Responsive | AML | Early trials |
| TP53 | A159V | Complete Match | Cisplatin (Chemotherapy) | Resistant | FGCT, MGCT | Early trials |
| TP53 | A159V | Complete Match | AZD6738 (ATR inhibitor) | Responsive | BCL | Early trials |
| TP53 | A159V | Complete Match | Gemcitabine (Chemotherapy) | Responsive | BLCA | Pre-clinical |
| TP53 | A159V | Complete Match | Mitomycin C (Chemotherapy) | Responsive | BLCA | Pre-clinical |
| TP53 | A159V | Complete Match | WEE1 inhibitor | Responsive | HNC | Pre-clinical |
| TP53 | A159V | Complete Match | MDM2 inhibitor | Resistant | LIP | Early trials |
| TP53 | A159V | Complete Match | Doxorubicin (Anthracycline antitumor antibiotic) | Responsive | BLCA | Pre-clinical |
| TP53 | A159V | Complete Match | Pramlintide (Amylin analogue) | Responsive | THYM | Pre-clinical |
| TP53 | A159V | Complete Match | WEE1 inhibitor | Responsive | OV | Early trials |
| TP53 | A159V | Different Alteration | Pramlintide (Amylin analogue) | Responsive | THYM | Pre-clinical |
| TP53 | A159V | Different Alteration | AZD6738 (ATR inhibitor) | Responsive | BCL | Early trials |
| TP53 | A138V | Different Mutation | HSP90 inhibitor | Responsive | CANCER | Pre-clinical |
| TP53 | A138V | Complete Match | Decitabine (Chemotherapy) | Responsive | AML, MDPS | Early trials |
| TP53 | A138V | Complete Match | Abemaciclib (CDK4/6 inhibitor) | Resistant | BRCA | Early trials |
| TP53 | A138V | Different Mutation | HDM2 inhibitor | Responsive | AML | Early trials |
| TP53 | A138V | Complete Match | Cisplatin (Chemotherapy) | Resistant | FGCT, MGCT | Early trials |
| TP53 | A138V | Complete Match | AZD6738 (ATR inhibitor) | Responsive | BCL | Early trials |
| TP53 | A138V | Complete Match | Gemcitabine (Chemotherapy) | Responsive | BLCA | Pre-clinical |
| TP53 | A138V | Complete Match | Mitomycin C (Chemotherapy) | Responsive | BLCA | Pre-clinical |
| TP53 | A138V | Complete Match | WEE1 inhibitor | Responsive | HNC | Pre-clinical |
| TP53 | A138V | Complete Match | MDM2 inhibitor | Resistant | LIP | Early trials |
| TP53 | A138V | Complete Match | Doxorubicin (Anthracycline antitumor antibiotic) | Responsive | BLCA | Pre-clinical |
| TP53 | A138V | Complete Match | Pramlintide (Amylin analogue) | Responsive | THYM | Pre-clinical |
| TP53 | A138V | Complete Match | WEE1 inhibitor | Responsive | OV | Early trials |
| TP53 | A138V | Different Alteration | Pramlintide (Amylin analogue) | Responsive | THYM | Pre-clinical |
| TP53 | A138V | Different Alteration | AZD6738 (ATR inhibitor) | Responsive | BCL | Early trials |
| TP53 | K132N | Different Mutation | HSP90 inhibitor | Responsive | CANCER | Pre-clinical |
| TP53 | K132N | Complete Match | Decitabine (Chemotherapy) | Responsive | AML, MDPS | Early trials |
| TP53 | K132N | Complete Match | Abemaciclib (CDK4/6 inhibitor) | Resistant | BRCA | Early trials |
| TP53 | K132N | Different Mutation | HDM2 inhibitor | Responsive | AML | Early trials |
| TP53 | K132N | Complete Match | Cisplatin (Chemotherapy) | Resistant | FGCT, MGCT | Early trials |
| TP53 | K132N | Complete Match | AZD6738 (ATR inhibitor) | Responsive | BCL | Early trials |
| TP53 | K132N | Complete Match | Gemcitabine (Chemotherapy) | Responsive | BLCA | Pre-clinical |
| TP53 | K132N | Complete Match | Mitomycin C (Chemotherapy) | Responsive | BLCA | Pre-clinical |
| TP53 | K132N | Complete Match | WEE1 inhibitor | Responsive | HNC | Pre-clinical |
| TP53 | K132N | Complete Match | MDM2 inhibitor | Resistant | LIP | Early trials |
| TP53 | K132N | Complete Match | Doxorubicin (Anthracycline antitumor antibiotic) | Responsive | BLCA | Pre-clinical |
| TP53 | K132N | Complete Match | Pramlintide (Amylin analogue) | Responsive | THYM | Pre-clinical |
| TP53 | K132N | Complete Match | WEE1 inhibitor | Responsive | OV | Early trials |
| TP53 | K132N | Different Alteration | Pramlintide (Amylin analogue) | Responsive | THYM | Pre-clinical |
| TP53 | K132N | Different Alteration | AZD6738 (ATR inhibitor) | Responsive | BCL | Early trials |
| TP53 | R273G | Different Mutation | HSP90 inhibitor | Responsive | CANCER | Pre-clinical |
| TP53 | R273G | Complete Match | Decitabine (Chemotherapy) | Responsive | AML, MDPS | Early trials |
| TP53 | R273G | Complete Match | Abemaciclib (CDK4/6 inhibitor) | Resistant | BRCA | Early trials |
| TP53 | R273G | Different Mutation | HDM2 inhibitor | Responsive | AML | Early trials |
| TP53 | R273G | Complete Match | Cisplatin (Chemotherapy) | Resistant | FGCT, MGCT | Early trials |
| TP53 | R273G | Complete Match | AZD6738 (ATR inhibitor) | Responsive | BCL | Early trials |
| TP53 | R273G | Complete Match | Gemcitabine (Chemotherapy) | Responsive | BLCA | Pre-clinical |
| TP53 | R273G | Complete Match | Mitomycin C (Chemotherapy) | Responsive | BLCA | Pre-clinical |
| TP53 | R273G | Complete Match | WEE1 inhibitor | Responsive | HNC | Pre-clinical |
| TP53 | R273G | Complete Match | MDM2 inhibitor | Resistant | LIP | Early trials |
| TP53 | R273G | Complete Match | Doxorubicin (Anthracycline antitumor antibiotic) | Responsive | BLCA | Pre-clinical |
| TP53 | R273G | Complete Match | Pramlintide (Amylin analogue) | Responsive | THYM | Pre-clinical |
| TP53 | R273G | Complete Match | WEE1 inhibitor | Responsive | OV | Early trials |
| TP53 | R273G | Different Alteration | Pramlintide (Amylin analogue) | Responsive | THYM | Pre-clinical |
| TP53 | R273G | Different Alteration | AZD6738 (ATR inhibitor) | Responsive | BCL | Early trials |
| TP53 | P278S | Different Mutation | HSP90 inhibitor | Responsive | CANCER | Pre-clinical |
| TP53 | P278S | Complete Match | Decitabine (Chemotherapy) | Responsive | AML, MDPS | Early trials |
| TP53 | P278S | Complete Match | Abemaciclib (CDK4/6 inhibitor) | Resistant | BRCA | Early trials |
| TP53 | P278S | Different Mutation | HDM2 inhibitor | Responsive | AML | Early trials |
| TP53 | P278S | Complete Match | Cisplatin (Chemotherapy) | Resistant | FGCT, MGCT | Early trials |
| TP53 | P278S | Complete Match | AZD6738 (ATR inhibitor) | Responsive | BCL | Early trials |
| TP53 | P278S | Complete Match | Gemcitabine (Chemotherapy) | Responsive | BLCA | Pre-clinical |
| TP53 | P278S | Complete Match | Mitomycin C (Chemotherapy) | Responsive | BLCA | Pre-clinical |
| TP53 | P278S | Complete Match | WEE1 inhibitor | Responsive | HNC | Pre-clinical |
| TP53 | P278S | Complete Match | MDM2 inhibitor | Resistant | LIP | Early trials |
| TP53 | P278S | Complete Match | Doxorubicin (Anthracycline antitumor antibiotic) | Responsive | BLCA | Pre-clinical |
| TP53 | P278S | Complete Match | Pramlintide (Amylin analogue) | Responsive | THYM | Pre-clinical |
| TP53 | P278S | Complete Match | WEE1 inhibitor | Responsive | OV | Early trials |
| TP53 | P278S | Different Alteration | Pramlintide (Amylin analogue) | Responsive | THYM | Pre-clinical |
| TP53 | P278S | Different Alteration | AZD6738 (ATR inhibitor) | Responsive | BCL | Early trials |
| TP53 | splice_donor_variant | Different Mutation | HSP90 inhibitor | Responsive | CANCER | Pre-clinical |
| TP53 | splice_donor_variant | Complete Match | Decitabine (Chemotherapy) | Responsive | AML, MDPS | Early trials |
| TP53 | splice_donor_variant | Complete Match | Abemaciclib (CDK4/6 inhibitor) | Resistant | BRCA | Early trials |
| TP53 | splice_donor_variant | Different Mutation | HDM2 inhibitor | Responsive | AML | Early trials |
| TP53 | splice_donor_variant | Complete Match | Cisplatin (Chemotherapy) | Resistant | FGCT, MGCT | Early trials |
| TP53 | splice_donor_variant | Complete Match | AZD6738 (ATR inhibitor) | Responsive | BCL | Early trials |
| TP53 | splice_donor_variant | Complete Match | Gemcitabine (Chemotherapy) | Responsive | BLCA | Pre-clinical |
| TP53 | splice_donor_variant | Complete Match | Mitomycin C (Chemotherapy) | Responsive | BLCA | Pre-clinical |
| TP53 | splice_donor_variant | Complete Match | WEE1 inhibitor | Responsive | HNC | Pre-clinical |
| TP53 | splice_donor_variant | Complete Match | MDM2 inhibitor | Resistant | LIP | Early trials |
| TP53 | splice_donor_variant | Complete Match | Doxorubicin (Anthracycline antitumor antibiotic) | Responsive | BLCA | Pre-clinical |
| TP53 | splice_donor_variant | Complete Match | Pramlintide (Amylin analogue) | Responsive | THYM | Pre-clinical |
| TP53 | splice_donor_variant | Complete Match | WEE1 inhibitor | Responsive | OV | Early trials |
| TP53 | splice_donor_variant | Different Alteration | Pramlintide (Amylin analogue) | Responsive | THYM | Pre-clinical |
| TP53 | splice_donor_variant | Different Alteration | AZD6738 (ATR inhibitor) | Responsive | BCL | Early trials |
| TP53 | R342* | Different Mutation | HSP90 inhibitor | Responsive | CANCER | Pre-clinical |
| TP53 | R342* | Complete Match | Decitabine (Chemotherapy) | Responsive | AML, MDPS | Early trials |
| TP53 | R342* | Complete Match | Abemaciclib (CDK4/6 inhibitor) | Resistant | BRCA | Early trials |
| TP53 | R342* | Different Mutation | HDM2 inhibitor | Responsive | AML | Early trials |
| TP53 | R342* | Complete Match | Cisplatin (Chemotherapy) | Resistant | FGCT, MGCT | Early trials |
| TP53 | R342* | Complete Match | AZD6738 (ATR inhibitor) | Responsive | BCL | Early trials |
| TP53 | R342* | Complete Match | Gemcitabine (Chemotherapy) | Responsive | BLCA | Pre-clinical |
| TP53 | R342* | Complete Match | Mitomycin C (Chemotherapy) | Responsive | BLCA | Pre-clinical |
| TP53 | R342* | Complete Match | WEE1 inhibitor | Responsive | HNC | Pre-clinical |
| TP53 | R342* | Complete Match | MDM2 inhibitor | Resistant | LIP | Early trials |
| TP53 | R342* | Complete Match | Doxorubicin (Anthracycline antitumor antibiotic) | Responsive | BLCA | Pre-clinical |
| TP53 | R342* | Complete Match | Pramlintide (Amylin analogue) | Responsive | THYM | Pre-clinical |
| TP53 | R342* | Complete Match | WEE1 inhibitor | Responsive | OV | Early trials |
| TP53 | R342* | Different Alteration | Pramlintide (Amylin analogue) | Responsive | THYM | Pre-clinical |
| TP53 | R342* | Different Alteration | AZD6738 (ATR inhibitor) | Responsive | BCL | Early trials |
| TP53 | D281G | Different Mutation | HSP90 inhibitor | Responsive | CANCER | Pre-clinical |
| TP53 | D281G | Complete Match | Decitabine (Chemotherapy) | Responsive | AML, MDPS | Early trials |
| TP53 | D281G | Complete Match | Abemaciclib (CDK4/6 inhibitor) | Resistant | BRCA | Early trials |
| TP53 | D281G | Different Mutation | HDM2 inhibitor | Responsive | AML | Early trials |
| TP53 | D281G | Complete Match | Cisplatin (Chemotherapy) | Resistant | FGCT, MGCT | Early trials |
| TP53 | D281G | Complete Match | AZD6738 (ATR inhibitor) | Responsive | BCL | Early trials |
| TP53 | D281G | Complete Match | Gemcitabine (Chemotherapy) | Responsive | BLCA | Pre-clinical |
| TP53 | D281G | Complete Match | Mitomycin C (Chemotherapy) | Responsive | BLCA | Pre-clinical |
| TP53 | D281G | Complete Match | WEE1 inhibitor | Responsive | HNC | Pre-clinical |
| TP53 | D281G | Complete Match | MDM2 inhibitor | Resistant | LIP | Early trials |
| TP53 | D281G | Complete Match | Doxorubicin (Anthracycline antitumor antibiotic) | Responsive | BLCA | Pre-clinical |
| TP53 | D281G | Complete Match | Pramlintide (Amylin analogue) | Responsive | THYM | Pre-clinical |
| TP53 | D281G | Complete Match | WEE1 inhibitor | Responsive | OV | Early trials |
| TP53 | D281G | Different Alteration | Pramlintide (Amylin analogue) | Responsive | THYM | Pre-clinical |
| TP53 | D281G | Different Alteration | AZD6738 (ATR inhibitor) | Responsive | BCL | Early trials |
| TP53 | T125T | Different Mutation | HSP90 inhibitor | Responsive | CANCER | Pre-clinical |
| TP53 | T125T | Complete Match | Decitabine (Chemotherapy) | Responsive | AML, MDPS | Early trials |
| TP53 | T125T | Complete Match | Abemaciclib (CDK4/6 inhibitor) | Resistant | BRCA | Early trials |
| TP53 | T125T | Different Mutation | HDM2 inhibitor | Responsive | AML | Early trials |
| TP53 | T125T | Complete Match | Cisplatin (Chemotherapy) | Resistant | FGCT, MGCT | Early trials |
| TP53 | T125T | Complete Match | AZD6738 (ATR inhibitor) | Responsive | BCL | Early trials |
| TP53 | T125T | Complete Match | Gemcitabine (Chemotherapy) | Responsive | BLCA | Pre-clinical |
| TP53 | T125T | Complete Match | Mitomycin C (Chemotherapy) | Responsive | BLCA | Pre-clinical |
| TP53 | T125T | Complete Match | WEE1 inhibitor | Responsive | HNC | Pre-clinical |
| TP53 | T125T | Complete Match | MDM2 inhibitor | Resistant | LIP | Early trials |
| TP53 | T125T | Complete Match | Doxorubicin (Anthracycline antitumor antibiotic) | Responsive | BLCA | Pre-clinical |
| TP53 | T125T | Complete Match | Pramlintide (Amylin analogue) | Responsive | THYM | Pre-clinical |
| TP53 | T125T | Complete Match | WEE1 inhibitor | Responsive | OV | Early trials |
| TP53 | T125T | Different Alteration | Pramlintide (Amylin analogue) | Responsive | THYM | Pre-clinical |
| TP53 | T125T | Different Alteration | AZD6738 (ATR inhibitor) | Responsive | BCL | Early trials |
| TP53 | Q192* | Different Mutation | HSP90 inhibitor | Responsive | CANCER | Pre-clinical |
| TP53 | Q192* | Complete Match | Decitabine (Chemotherapy) | Responsive | AML, MDPS | Early trials |
| TP53 | Q192* | Complete Match | Abemaciclib (CDK4/6 inhibitor) | Resistant | BRCA | Early trials |
| TP53 | Q192* | Different Mutation | HDM2 inhibitor | Responsive | AML | Early trials |
| TP53 | Q192* | Complete Match | Cisplatin (Chemotherapy) | Resistant | FGCT, MGCT | Early trials |
| TP53 | Q192* | Complete Match | AZD6738 (ATR inhibitor) | Responsive | BCL | Early trials |
| TP53 | Q192* | Complete Match | Gemcitabine (Chemotherapy) | Responsive | BLCA | Pre-clinical |
| TP53 | Q192* | Complete Match | Mitomycin C (Chemotherapy) | Responsive | BLCA | Pre-clinical |
| TP53 | Q192* | Complete Match | WEE1 inhibitor | Responsive | HNC | Pre-clinical |
| TP53 | Q192* | Complete Match | MDM2 inhibitor | Resistant | LIP | Early trials |
| TP53 | Q192* | Complete Match | Doxorubicin (Anthracycline antitumor antibiotic) | Responsive | BLCA | Pre-clinical |
| TP53 | Q192* | Complete Match | Pramlintide (Amylin analogue) | Responsive | THYM | Pre-clinical |
| TP53 | Q192* | Complete Match | WEE1 inhibitor | Responsive | OV | Early trials |
| TP53 | Q192* | Different Alteration | Pramlintide (Amylin analogue) | Responsive | THYM | Pre-clinical |
| TP53 | Q192* | Different Alteration | AZD6738 (ATR inhibitor) | Responsive | BCL | Early trials |
| TP53 | R248L | Different Mutation | HSP90 inhibitor | Responsive | CANCER | Pre-clinical |
| TP53 | R248L | Complete Match | Decitabine (Chemotherapy) | Responsive | AML, MDPS | Early trials |
| TP53 | R248L | Complete Match | Abemaciclib (CDK4/6 inhibitor) | Resistant | BRCA | Early trials |
| TP53 | R248L | Different Mutation | HDM2 inhibitor | Responsive | AML | Early trials |
| TP53 | R248L | Complete Match | Cisplatin (Chemotherapy) | Resistant | FGCT, MGCT | Early trials |
| TP53 | R248L | Complete Match | AZD6738 (ATR inhibitor) | Responsive | BCL | Early trials |
| TP53 | R248L | Complete Match | Gemcitabine (Chemotherapy) | Responsive | BLCA | Pre-clinical |
| TP53 | R248L | Complete Match | Mitomycin C (Chemotherapy) | Responsive | BLCA | Pre-clinical |
| TP53 | R248L | Complete Match | WEE1 inhibitor | Responsive | HNC | Pre-clinical |
| TP53 | R248L | Complete Match | MDM2 inhibitor | Resistant | LIP | Early trials |
| TP53 | R248L | Complete Match | Doxorubicin (Anthracycline antitumor antibiotic) | Responsive | BLCA | Pre-clinical |
| TP53 | R248L | Complete Match | Pramlintide (Amylin analogue) | Responsive | THYM | Pre-clinical |
| TP53 | R248L | Complete Match | WEE1 inhibitor | Responsive | OV | Early trials |
| TP53 | R248L | Different Alteration | Pramlintide (Amylin analogue) | Responsive | THYM | Pre-clinical |
| TP53 | R248L | Different Alteration | AZD6738 (ATR inhibitor) | Responsive | BCL | Early trials |
| TP53 | E68* | Different Mutation | HSP90 inhibitor | Responsive | CANCER | Pre-clinical |
| TP53 | E68* | Complete Match | Decitabine (Chemotherapy) | Responsive | AML, MDPS | Early trials |
| TP53 | E68* | Complete Match | Abemaciclib (CDK4/6 inhibitor) | Resistant | BRCA | Early trials |
| TP53 | E68* | Different Mutation | HDM2 inhibitor | Responsive | AML | Early trials |
| TP53 | E68* | Complete Match | Cisplatin (Chemotherapy) | Resistant | FGCT, MGCT | Early trials |
| TP53 | E68* | Complete Match | AZD6738 (ATR inhibitor) | Responsive | BCL | Early trials |
| TP53 | E68* | Complete Match | Gemcitabine (Chemotherapy) | Responsive | BLCA | Pre-clinical |
| TP53 | E68* | Complete Match | Mitomycin C (Chemotherapy) | Responsive | BLCA | Pre-clinical |
| TP53 | E68* | Complete Match | WEE1 inhibitor | Responsive | HNC | Pre-clinical |
| TP53 | E68* | Complete Match | MDM2 inhibitor | Resistant | LIP | Early trials |
| TP53 | E68* | Complete Match | Doxorubicin (Anthracycline antitumor antibiotic) | Responsive | BLCA | Pre-clinical |
| TP53 | E68* | Complete Match | Pramlintide (Amylin analogue) | Responsive | THYM | Pre-clinical |
| TP53 | E68* | Complete Match | WEE1 inhibitor | Responsive | OV | Early trials |
| TP53 | E68* | Different Alteration | Pramlintide (Amylin analogue) | Responsive | THYM | Pre-clinical |
| TP53 | E68* | Different Alteration | AZD6738 (ATR inhibitor) | Responsive | BCL | Early trials |
| TP53 | Y205C | Different Mutation | HSP90 inhibitor | Responsive | CANCER | Pre-clinical |
| TP53 | Y205C | Complete Match | Decitabine (Chemotherapy) | Responsive | AML, MDPS | Early trials |
| TP53 | Y205C | Complete Match | Abemaciclib (CDK4/6 inhibitor) | Resistant | BRCA | Early trials |
| TP53 | Y205C | Different Mutation | HDM2 inhibitor | Responsive | AML | Early trials |
| TP53 | Y205C | Complete Match | Cisplatin (Chemotherapy) | Resistant | FGCT, MGCT | Early trials |
| TP53 | Y205C | Complete Match | AZD6738 (ATR inhibitor) | Responsive | BCL | Early trials |
| TP53 | Y205C | Complete Match | Gemcitabine (Chemotherapy) | Responsive | BLCA | Pre-clinical |
| TP53 | Y205C | Complete Match | Mitomycin C (Chemotherapy) | Responsive | BLCA | Pre-clinical |
| TP53 | Y205C | Complete Match | WEE1 inhibitor | Responsive | HNC | Pre-clinical |
| TP53 | Y205C | Complete Match | MDM2 inhibitor | Resistant | LIP | Early trials |
| TP53 | Y205C | Complete Match | Doxorubicin (Anthracycline antitumor antibiotic) | Responsive | BLCA | Pre-clinical |
| TP53 | Y205C | Complete Match | Pramlintide (Amylin analogue) | Responsive | THYM | Pre-clinical |
| TP53 | Y205C | Complete Match | WEE1 inhibitor | Responsive | OV | Early trials |
| TP53 | Y205C | Different Alteration | Pramlintide (Amylin analogue) | Responsive | THYM | Pre-clinical |
| TP53 | Y205C | Different Alteration | AZD6738 (ATR inhibitor) | Responsive | BCL | Early trials |
| TP53 | M237I | Different Mutation | HSP90 inhibitor | Responsive | CANCER | Pre-clinical |
| TP53 | M237I | Complete Match | Decitabine (Chemotherapy) | Responsive | AML, MDPS | Early trials |
| TP53 | M237I | Complete Match | Abemaciclib (CDK4/6 inhibitor) | Resistant | BRCA | Early trials |
| TP53 | M237I | Different Mutation | HDM2 inhibitor | Responsive | AML | Early trials |
| TP53 | M237I | Complete Match | Cisplatin (Chemotherapy) | Resistant | FGCT, MGCT | Early trials |
| TP53 | M237I | Complete Match | AZD6738 (ATR inhibitor) | Responsive | BCL | Early trials |
| TP53 | M237I | Complete Match | Gemcitabine (Chemotherapy) | Responsive | BLCA | Pre-clinical |
| TP53 | M237I | Complete Match | Mitomycin C (Chemotherapy) | Responsive | BLCA | Pre-clinical |
| TP53 | M237I | Complete Match | WEE1 inhibitor | Responsive | HNC | Pre-clinical |
| TP53 | M237I | Complete Match | MDM2 inhibitor | Resistant | LIP | Early trials |
| TP53 | M237I | Complete Match | Doxorubicin (Anthracycline antitumor antibiotic) | Responsive | BLCA | Pre-clinical |
| TP53 | M237I | Complete Match | Pramlintide (Amylin analogue) | Responsive | THYM | Pre-clinical |
| TP53 | M237I | Complete Match | WEE1 inhibitor | Responsive | OV | Early trials |
| TP53 | M237I | Different Alteration | Pramlintide (Amylin analogue) | Responsive | THYM | Pre-clinical |
| TP53 | M237I | Different Alteration | AZD6738 (ATR inhibitor) | Responsive | BCL | Early trials |
| TP53 | V172F | Different Mutation | HSP90 inhibitor | Responsive | CANCER | Pre-clinical |
| TP53 | V172F | Complete Match | Decitabine (Chemotherapy) | Responsive | AML, MDPS | Early trials |
| TP53 | V172F | Complete Match | Abemaciclib (CDK4/6 inhibitor) | Resistant | BRCA | Early trials |
| TP53 | V172F | Different Mutation | HDM2 inhibitor | Responsive | AML | Early trials |
| TP53 | V172F | Complete Match | Cisplatin (Chemotherapy) | Resistant | FGCT, MGCT | Early trials |
| TP53 | V172F | Complete Match | AZD6738 (ATR inhibitor) | Responsive | BCL | Early trials |
| TP53 | V172F | Complete Match | Gemcitabine (Chemotherapy) | Responsive | BLCA | Pre-clinical |
| TP53 | V172F | Complete Match | Mitomycin C (Chemotherapy) | Responsive | BLCA | Pre-clinical |
| TP53 | V172F | Complete Match | WEE1 inhibitor | Responsive | HNC | Pre-clinical |
| TP53 | V172F | Complete Match | MDM2 inhibitor | Resistant | LIP | Early trials |
| TP53 | V172F | Complete Match | Doxorubicin (Anthracycline antitumor antibiotic) | Responsive | BLCA | Pre-clinical |
| TP53 | V172F | Complete Match | Pramlintide (Amylin analogue) | Responsive | THYM | Pre-clinical |
| TP53 | V172F | Complete Match | WEE1 inhibitor | Responsive | OV | Early trials |
| TP53 | V172F | Different Alteration | Pramlintide (Amylin analogue) | Responsive | THYM | Pre-clinical |
| TP53 | V172F | Different Alteration | AZD6738 (ATR inhibitor) | Responsive | BCL | Early trials |
| TP53 | V217G | Different Mutation | HSP90 inhibitor | Responsive | CANCER | Pre-clinical |
| TP53 | V217G | Complete Match | Decitabine (Chemotherapy) | Responsive | AML, MDPS | Early trials |
| TP53 | V217G | Complete Match | Abemaciclib (CDK4/6 inhibitor) | Resistant | BRCA | Early trials |
| TP53 | V217G | Different Mutation | HDM2 inhibitor | Responsive | AML | Early trials |
| TP53 | V217G | Complete Match | Cisplatin (Chemotherapy) | Resistant | FGCT, MGCT | Early trials |
| TP53 | V217G | Complete Match | AZD6738 (ATR inhibitor) | Responsive | BCL | Early trials |
| TP53 | V217G | Complete Match | Gemcitabine (Chemotherapy) | Responsive | BLCA | Pre-clinical |
| TP53 | V217G | Complete Match | Mitomycin C (Chemotherapy) | Responsive | BLCA | Pre-clinical |
| TP53 | V217G | Complete Match | WEE1 inhibitor | Responsive | HNC | Pre-clinical |
| TP53 | V217G | Complete Match | MDM2 inhibitor | Resistant | LIP | Early trials |
| TP53 | V217G | Complete Match | Doxorubicin (Anthracycline antitumor antibiotic) | Responsive | BLCA | Pre-clinical |
| TP53 | V217G | Complete Match | Pramlintide (Amylin analogue) | Responsive | THYM | Pre-clinical |
| TP53 | V217G | Complete Match | WEE1 inhibitor | Responsive | OV | Early trials |
| TP53 | V217G | Different Alteration | Pramlintide (Amylin analogue) | Responsive | THYM | Pre-clinical |
| TP53 | V217G | Different Alteration | AZD6738 (ATR inhibitor) | Responsive | BCL | Early trials |
| TP53 | C176S | Different Mutation | HSP90 inhibitor | Responsive | CANCER | Pre-clinical |
| TP53 | C176S | Complete Match | Decitabine (Chemotherapy) | Responsive | AML, MDPS | Early trials |
| TP53 | C176S | Complete Match | Abemaciclib (CDK4/6 inhibitor) | Resistant | BRCA | Early trials |
| TP53 | C176S | Different Mutation | HDM2 inhibitor | Responsive | AML | Early trials |
| TP53 | C176S | Complete Match | Cisplatin (Chemotherapy) | Resistant | FGCT, MGCT | Early trials |
| TP53 | C176S | Complete Match | AZD6738 (ATR inhibitor) | Responsive | BCL | Early trials |
| TP53 | C176S | Complete Match | Gemcitabine (Chemotherapy) | Responsive | BLCA | Pre-clinical |
| TP53 | C176S | Complete Match | Mitomycin C (Chemotherapy) | Responsive | BLCA | Pre-clinical |
| TP53 | C176S | Complete Match | WEE1 inhibitor | Responsive | HNC | Pre-clinical |
| TP53 | C176S | Complete Match | MDM2 inhibitor | Resistant | LIP | Early trials |
| TP53 | C176S | Complete Match | Doxorubicin (Anthracycline antitumor antibiotic) | Responsive | BLCA | Pre-clinical |
| TP53 | C176S | Complete Match | Pramlintide (Amylin analogue) | Responsive | THYM | Pre-clinical |
| TP53 | C176S | Complete Match | WEE1 inhibitor | Responsive | OV | Early trials |
| TP53 | C176S | Different Alteration | Pramlintide (Amylin analogue) | Responsive | THYM | Pre-clinical |
| TP53 | C176S | Different Alteration | AZD6738 (ATR inhibitor) | Responsive | BCL | Early trials |
| TP53 | L145Q | Different Mutation | HSP90 inhibitor | Responsive | CANCER | Pre-clinical |
| TP53 | L145Q | Complete Match | Decitabine (Chemotherapy) | Responsive | AML, MDPS | Early trials |
| TP53 | L145Q | Complete Match | Abemaciclib (CDK4/6 inhibitor) | Resistant | BRCA | Early trials |
| TP53 | L145Q | Different Mutation | HDM2 inhibitor | Responsive | AML | Early trials |
| TP53 | L145Q | Complete Match | Cisplatin (Chemotherapy) | Resistant | FGCT, MGCT | Early trials |
| TP53 | L145Q | Complete Match | AZD6738 (ATR inhibitor) | Responsive | BCL | Early trials |
| TP53 | L145Q | Complete Match | Gemcitabine (Chemotherapy) | Responsive | BLCA | Pre-clinical |
| TP53 | L145Q | Complete Match | Mitomycin C (Chemotherapy) | Responsive | BLCA | Pre-clinical |
| TP53 | L145Q | Complete Match | WEE1 inhibitor | Responsive | HNC | Pre-clinical |
| TP53 | L145Q | Complete Match | MDM2 inhibitor | Resistant | LIP | Early trials |
| TP53 | L145Q | Complete Match | Doxorubicin (Anthracycline antitumor antibiotic) | Responsive | BLCA | Pre-clinical |
| TP53 | L145Q | Complete Match | Pramlintide (Amylin analogue) | Responsive | THYM | Pre-clinical |
| TP53 | L145Q | Complete Match | WEE1 inhibitor | Responsive | OV | Early trials |
| TP53 | L145Q | Different Alteration | Pramlintide (Amylin analogue) | Responsive | THYM | Pre-clinical |
| TP53 | L145Q | Different Alteration | AZD6738 (ATR inhibitor) | Responsive | BCL | Early trials |
| TP53 | Q317* | Different Mutation | HSP90 inhibitor | Responsive | CANCER | Pre-clinical |
| TP53 | Q317* | Complete Match | Decitabine (Chemotherapy) | Responsive | AML, MDPS | Early trials |
| TP53 | Q317* | Complete Match | Abemaciclib (CDK4/6 inhibitor) | Resistant | BRCA | Early trials |
| TP53 | Q317* | Different Mutation | HDM2 inhibitor | Responsive | AML | Early trials |
| TP53 | Q317* | Complete Match | Cisplatin (Chemotherapy) | Resistant | FGCT, MGCT | Early trials |
| TP53 | Q317* | Complete Match | AZD6738 (ATR inhibitor) | Responsive | BCL | Early trials |
| TP53 | Q317* | Complete Match | Gemcitabine (Chemotherapy) | Responsive | BLCA | Pre-clinical |
| TP53 | Q317* | Complete Match | Mitomycin C (Chemotherapy) | Responsive | BLCA | Pre-clinical |
| TP53 | Q317* | Complete Match | WEE1 inhibitor | Responsive | HNC | Pre-clinical |
| TP53 | Q317* | Complete Match | MDM2 inhibitor | Resistant | LIP | Early trials |
| TP53 | Q317* | Complete Match | Doxorubicin (Anthracycline antitumor antibiotic) | Responsive | BLCA | Pre-clinical |
| TP53 | Q317* | Complete Match | Pramlintide (Amylin analogue) | Responsive | THYM | Pre-clinical |
| TP53 | Q317* | Complete Match | WEE1 inhibitor | Responsive | OV | Early trials |
| TP53 | Q317* | Different Alteration | Pramlintide (Amylin analogue) | Responsive | THYM | Pre-clinical |
| TP53 | Q317* | Different Alteration | AZD6738 (ATR inhibitor) | Responsive | BCL | Early trials |
| TP53 | C176F | Different Mutation | HSP90 inhibitor | Responsive | CANCER | Pre-clinical |
| TP53 | C176F | Complete Match | Decitabine (Chemotherapy) | Responsive | AML, MDPS | Early trials |
| TP53 | C176F | Complete Match | Abemaciclib (CDK4/6 inhibitor) | Resistant | BRCA | Early trials |
| TP53 | C176F | Different Mutation | HDM2 inhibitor | Responsive | AML | Early trials |
| TP53 | C176F | Complete Match | Cisplatin (Chemotherapy) | Resistant | FGCT, MGCT | Early trials |
| TP53 | C176F | Complete Match | AZD6738 (ATR inhibitor) | Responsive | BCL | Early trials |
| TP53 | C176F | Complete Match | Gemcitabine (Chemotherapy) | Responsive | BLCA | Pre-clinical |
| TP53 | C176F | Complete Match | Mitomycin C (Chemotherapy) | Responsive | BLCA | Pre-clinical |
| TP53 | C176F | Complete Match | WEE1 inhibitor | Responsive | HNC | Pre-clinical |
| TP53 | C176F | Complete Match | MDM2 inhibitor | Resistant | LIP | Early trials |
| TP53 | C176F | Complete Match | Doxorubicin (Anthracycline antitumor antibiotic) | Responsive | BLCA | Pre-clinical |
| TP53 | C176F | Complete Match | Pramlintide (Amylin analogue) | Responsive | THYM | Pre-clinical |
| TP53 | C176F | Complete Match | WEE1 inhibitor | Responsive | OV | Early trials |
| TP53 | C176F | Different Alteration | Pramlintide (Amylin analogue) | Responsive | THYM | Pre-clinical |
| TP53 | C176F | Different Alteration | AZD6738 (ATR inhibitor) | Responsive | BCL | Early trials |
| TP53 | A161T | Different Mutation | HSP90 inhibitor | Responsive | CANCER | Pre-clinical |
| TP53 | A161T | Complete Match | Decitabine (Chemotherapy) | Responsive | AML, MDPS | Early trials |
| TP53 | A161T | Complete Match | Abemaciclib (CDK4/6 inhibitor) | Resistant | BRCA | Early trials |
| TP53 | A161T | Different Mutation | HDM2 inhibitor | Responsive | AML | Early trials |
| TP53 | A161T | Complete Match | Cisplatin (Chemotherapy) | Resistant | FGCT, MGCT | Early trials |
| TP53 | A161T | Complete Match | AZD6738 (ATR inhibitor) | Responsive | BCL | Early trials |
| TP53 | A161T | Complete Match | Gemcitabine (Chemotherapy) | Responsive | BLCA | Pre-clinical |
| TP53 | A161T | Complete Match | Mitomycin C (Chemotherapy) | Responsive | BLCA | Pre-clinical |
| TP53 | A161T | Complete Match | WEE1 inhibitor | Responsive | HNC | Pre-clinical |
| TP53 | A161T | Complete Match | MDM2 inhibitor | Resistant | LIP | Early trials |
| TP53 | A161T | Complete Match | Doxorubicin (Anthracycline antitumor antibiotic) | Responsive | BLCA | Pre-clinical |
| TP53 | A161T | Complete Match | Pramlintide (Amylin analogue) | Responsive | THYM | Pre-clinical |
| TP53 | A161T | Complete Match | WEE1 inhibitor | Responsive | OV | Early trials |
| TP53 | A161T | Different Alteration | Pramlintide (Amylin analogue) | Responsive | THYM | Pre-clinical |
| TP53 | A161T | Different Alteration | AZD6738 (ATR inhibitor) | Responsive | BCL | Early trials |
| TP53 | W91* | Different Mutation | HSP90 inhibitor | Responsive | CANCER | Pre-clinical |
| TP53 | W91* | Complete Match | Decitabine (Chemotherapy) | Responsive | AML, MDPS | Early trials |
| TP53 | W91* | Complete Match | Abemaciclib (CDK4/6 inhibitor) | Resistant | BRCA | Early trials |
| TP53 | W91* | Different Mutation | HDM2 inhibitor | Responsive | AML | Early trials |
| TP53 | W91* | Complete Match | Cisplatin (Chemotherapy) | Resistant | FGCT, MGCT | Early trials |
| TP53 | W91* | Complete Match | AZD6738 (ATR inhibitor) | Responsive | BCL | Early trials |
| TP53 | W91* | Complete Match | Gemcitabine (Chemotherapy) | Responsive | BLCA | Pre-clinical |
| TP53 | W91* | Complete Match | Mitomycin C (Chemotherapy) | Responsive | BLCA | Pre-clinical |
| TP53 | W91* | Complete Match | WEE1 inhibitor | Responsive | HNC | Pre-clinical |
| TP53 | W91* | Complete Match | MDM2 inhibitor | Resistant | LIP | Early trials |
| TP53 | W91* | Complete Match | Doxorubicin (Anthracycline antitumor antibiotic) | Responsive | BLCA | Pre-clinical |
| TP53 | W91* | Complete Match | Pramlintide (Amylin analogue) | Responsive | THYM | Pre-clinical |
| TP53 | W91* | Complete Match | WEE1 inhibitor | Responsive | OV | Early trials |
| TP53 | W91* | Different Alteration | Pramlintide (Amylin analogue) | Responsive | THYM | Pre-clinical |
| TP53 | W91* | Different Alteration | AZD6738 (ATR inhibitor) | Responsive | BCL | Early trials |
| TP53 | D259V | Different Mutation | HSP90 inhibitor | Responsive | CANCER | Pre-clinical |
| TP53 | D259V | Complete Match | Decitabine (Chemotherapy) | Responsive | AML, MDPS | Early trials |
| TP53 | D259V | Complete Match | Abemaciclib (CDK4/6 inhibitor) | Resistant | BRCA | Early trials |
| TP53 | D259V | Different Mutation | HDM2 inhibitor | Responsive | AML | Early trials |
| TP53 | D259V | Complete Match | Cisplatin (Chemotherapy) | Resistant | FGCT, MGCT | Early trials |
| TP53 | D259V | Complete Match | AZD6738 (ATR inhibitor) | Responsive | BCL | Early trials |
| TP53 | D259V | Complete Match | Gemcitabine (Chemotherapy) | Responsive | BLCA | Pre-clinical |
| TP53 | D259V | Complete Match | Mitomycin C (Chemotherapy) | Responsive | BLCA | Pre-clinical |
| TP53 | D259V | Complete Match | WEE1 inhibitor | Responsive | HNC | Pre-clinical |
| TP53 | D259V | Complete Match | MDM2 inhibitor | Resistant | LIP | Early trials |
| TP53 | D259V | Complete Match | Doxorubicin (Anthracycline antitumor antibiotic) | Responsive | BLCA | Pre-clinical |
| TP53 | D259V | Complete Match | Pramlintide (Amylin analogue) | Responsive | THYM | Pre-clinical |
| TP53 | D259V | Complete Match | WEE1 inhibitor | Responsive | OV | Early trials |
| TP53 | D259V | Different Alteration | Pramlintide (Amylin analogue) | Responsive | THYM | Pre-clinical |
| TP53 | D259V | Different Alteration | AZD6738 (ATR inhibitor) | Responsive | BCL | Early trials |
| TP53 | R158H | Different Mutation | HSP90 inhibitor | Responsive | CANCER | Pre-clinical |
| TP53 | R158H | Complete Match | Decitabine (Chemotherapy) | Responsive | AML, MDPS | Early trials |
| TP53 | R158H | Complete Match | Abemaciclib (CDK4/6 inhibitor) | Resistant | BRCA | Early trials |
| TP53 | R158H | Different Mutation | HDM2 inhibitor | Responsive | AML | Early trials |
| TP53 | R158H | Complete Match | Cisplatin (Chemotherapy) | Resistant | FGCT, MGCT | Early trials |
| TP53 | R158H | Complete Match | AZD6738 (ATR inhibitor) | Responsive | BCL | Early trials |
| TP53 | R158H | Complete Match | Gemcitabine (Chemotherapy) | Responsive | BLCA | Pre-clinical |
| TP53 | R158H | Complete Match | Mitomycin C (Chemotherapy) | Responsive | BLCA | Pre-clinical |
| TP53 | R158H | Complete Match | WEE1 inhibitor | Responsive | HNC | Pre-clinical |
| TP53 | R158H | Complete Match | MDM2 inhibitor | Resistant | LIP | Early trials |
| TP53 | R158H | Complete Match | Doxorubicin (Anthracycline antitumor antibiotic) | Responsive | BLCA | Pre-clinical |
| TP53 | R158H | Complete Match | Pramlintide (Amylin analogue) | Responsive | THYM | Pre-clinical |
| TP53 | R158H | Complete Match | WEE1 inhibitor | Responsive | OV | Early trials |
| TP53 | R158H | Different Alteration | Pramlintide (Amylin analogue) | Responsive | THYM | Pre-clinical |
| TP53 | R158H | Different Alteration | AZD6738 (ATR inhibitor) | Responsive | BCL | Early trials |
| TP53 | R248Q), TP53 MUT (D281E | Complete Match | Decitabine (Chemotherapy) | Responsive | AML, MDPS | Early trials |
| TP53 | R248Q), TP53 MUT (D281E | Complete Match | Abemaciclib (CDK4/6 inhibitor) | Resistant | BRCA | Early trials |
| TP53 | R248Q), TP53 MUT (D281E | Different Mutation | HDM2 inhibitor | Responsive | AML | Early trials |
| TP53 | R248Q), TP53 MUT (D281E | Complete Match | Cisplatin (Chemotherapy) | Resistant | FGCT, MGCT | Early trials |
| TP53 | R248Q), TP53 MUT (D281E | Complete Match | AZD6738 (ATR inhibitor) | Responsive | BCL | Early trials |
| TP53 | R248Q), TP53 MUT (D281E | Complete Match | Gemcitabine (Chemotherapy) | Responsive | BLCA | Pre-clinical |
| TP53 | R248Q), TP53 MUT (D281E | Complete Match | Mitomycin C (Chemotherapy) | Responsive | BLCA | Pre-clinical |
| TP53 | R248Q), TP53 MUT (D281E | Complete Match | WEE1 inhibitor | Responsive | HNC | Pre-clinical |
| TP53 | R248Q), TP53 MUT (D281E | Complete Match | MDM2 inhibitor | Resistant | LIP | Early trials |
| TP53 | R248Q), TP53 MUT (D281E | Complete Match | Doxorubicin (Anthracycline antitumor antibiotic) | Responsive | BLCA | Pre-clinical |
| TP53 | R248Q), TP53 MUT (D281E | Complete Match | Pramlintide (Amylin analogue) | Responsive | THYM | Pre-clinical |
| TP53 | R248Q), TP53 MUT (D281E | Complete Match | WEE1 inhibitor | Responsive | OV | Early trials |
| TP53 | R248Q), TP53 MUT (D281E | Different Alteration | Pramlintide (Amylin analogue) | Responsive | THYM | Pre-clinical |
| TP53 | R248Q), TP53 MUT (D281E | Different Alteration | AZD6738 (ATR inhibitor) | Responsive | BCL | Early trials |
| TP53 | G262V | Different Mutation | HSP90 inhibitor | Responsive | CANCER | Pre-clinical |
| TP53 | G262V | Complete Match | Decitabine (Chemotherapy) | Responsive | AML, MDPS | Early trials |
| TP53 | G262V | Complete Match | Abemaciclib (CDK4/6 inhibitor) | Resistant | BRCA | Early trials |
| TP53 | G262V | Different Mutation | HDM2 inhibitor | Responsive | AML | Early trials |
| TP53 | G262V | Complete Match | Cisplatin (Chemotherapy) | Resistant | FGCT, MGCT | Early trials |
| TP53 | G262V | Complete Match | AZD6738 (ATR inhibitor) | Responsive | BCL | Early trials |
| TP53 | G262V | Complete Match | Gemcitabine (Chemotherapy) | Responsive | BLCA | Pre-clinical |
| TP53 | G262V | Complete Match | Mitomycin C (Chemotherapy) | Responsive | BLCA | Pre-clinical |
| TP53 | G262V | Complete Match | WEE1 inhibitor | Responsive | HNC | Pre-clinical |
| TP53 | G262V | Complete Match | MDM2 inhibitor | Resistant | LIP | Early trials |
| TP53 | G262V | Complete Match | Doxorubicin (Anthracycline antitumor antibiotic) | Responsive | BLCA | Pre-clinical |
| TP53 | G262V | Complete Match | Pramlintide (Amylin analogue) | Responsive | THYM | Pre-clinical |
| TP53 | G262V | Complete Match | WEE1 inhibitor | Responsive | OV | Early trials |
| TP53 | G262V | Different Alteration | Pramlintide (Amylin analogue) | Responsive | THYM | Pre-clinical |
| TP53 | G262V | Different Alteration | AZD6738 (ATR inhibitor) | Responsive | BCL | Early trials |
| TP53 | R280G | Different Mutation | HSP90 inhibitor | Responsive | CANCER | Pre-clinical |
| TP53 | R280G | Complete Match | Decitabine (Chemotherapy) | Responsive | AML, MDPS | Early trials |
| TP53 | R280G | Complete Match | Abemaciclib (CDK4/6 inhibitor) | Resistant | BRCA | Early trials |
| TP53 | R280G | Different Mutation | HDM2 inhibitor | Responsive | AML | Early trials |
| TP53 | R280G | Complete Match | Cisplatin (Chemotherapy) | Resistant | FGCT, MGCT | Early trials |
| TP53 | R280G | Complete Match | AZD6738 (ATR inhibitor) | Responsive | BCL | Early trials |
| TP53 | R280G | Complete Match | Gemcitabine (Chemotherapy) | Responsive | BLCA | Pre-clinical |
| TP53 | R280G | Complete Match | Mitomycin C (Chemotherapy) | Responsive | BLCA | Pre-clinical |
| TP53 | R280G | Complete Match | WEE1 inhibitor | Responsive | HNC | Pre-clinical |
| TP53 | R280G | Complete Match | MDM2 inhibitor | Resistant | LIP | Early trials |
| TP53 | R280G | Complete Match | Doxorubicin (Anthracycline antitumor antibiotic) | Responsive | BLCA | Pre-clinical |
| TP53 | R280G | Complete Match | Pramlintide (Amylin analogue) | Responsive | THYM | Pre-clinical |
| TP53 | R280G | Complete Match | WEE1 inhibitor | Responsive | OV | Early trials |
| TP53 | R280G | Different Alteration | Pramlintide (Amylin analogue) | Responsive | THYM | Pre-clinical |
| TP53 | R280G | Different Alteration | AZD6738 (ATR inhibitor) | Responsive | BCL | Early trials |
| TP53 | G266E | Different Mutation | HSP90 inhibitor | Responsive | CANCER | Pre-clinical |
| TP53 | G266E | Complete Match | Decitabine (Chemotherapy) | Responsive | AML, MDPS | Early trials |
| TP53 | G266E | Complete Match | Abemaciclib (CDK4/6 inhibitor) | Resistant | BRCA | Early trials |
| TP53 | G266E | Different Mutation | HDM2 inhibitor | Responsive | AML | Early trials |
| TP53 | G266E | Complete Match | Cisplatin (Chemotherapy) | Resistant | FGCT, MGCT | Early trials |
| TP53 | G266E | Complete Match | AZD6738 (ATR inhibitor) | Responsive | BCL | Early trials |
| TP53 | G266E | Complete Match | Gemcitabine (Chemotherapy) | Responsive | BLCA | Pre-clinical |
| TP53 | G266E | Complete Match | Mitomycin C (Chemotherapy) | Responsive | BLCA | Pre-clinical |
| TP53 | G266E | Complete Match | WEE1 inhibitor | Responsive | HNC | Pre-clinical |
| TP53 | G266E | Complete Match | MDM2 inhibitor | Resistant | LIP | Early trials |
| TP53 | G266E | Complete Match | Doxorubicin (Anthracycline antitumor antibiotic) | Responsive | BLCA | Pre-clinical |
| TP53 | G266E | Complete Match | Pramlintide (Amylin analogue) | Responsive | THYM | Pre-clinical |
| TP53 | G266E | Complete Match | WEE1 inhibitor | Responsive | OV | Early trials |
| TP53 | G266E | Different Alteration | Pramlintide (Amylin analogue) | Responsive | THYM | Pre-clinical |
| TP53 | G266E | Different Alteration | AZD6738 (ATR inhibitor) | Responsive | BCL | Early trials |
| TP53 | C275G | Different Mutation | HSP90 inhibitor | Responsive | CANCER | Pre-clinical |
| TP53 | C275G | Complete Match | Decitabine (Chemotherapy) | Responsive | AML, MDPS | Early trials |
| TP53 | C275G | Complete Match | Abemaciclib (CDK4/6 inhibitor) | Resistant | BRCA | Early trials |
| TP53 | C275G | Different Mutation | HDM2 inhibitor | Responsive | AML | Early trials |
| TP53 | C275G | Complete Match | Cisplatin (Chemotherapy) | Resistant | FGCT, MGCT | Early trials |
| TP53 | C275G | Complete Match | AZD6738 (ATR inhibitor) | Responsive | BCL | Early trials |
| TP53 | C275G | Complete Match | Gemcitabine (Chemotherapy) | Responsive | BLCA | Pre-clinical |
| TP53 | C275G | Complete Match | Mitomycin C (Chemotherapy) | Responsive | BLCA | Pre-clinical |
| TP53 | C275G | Complete Match | WEE1 inhibitor | Responsive | HNC | Pre-clinical |
| TP53 | C275G | Complete Match | MDM2 inhibitor | Resistant | LIP | Early trials |
| TP53 | C275G | Complete Match | Doxorubicin (Anthracycline antitumor antibiotic) | Responsive | BLCA | Pre-clinical |
| TP53 | C275G | Complete Match | Pramlintide (Amylin analogue) | Responsive | THYM | Pre-clinical |
| TP53 | C275G | Complete Match | WEE1 inhibitor | Responsive | OV | Early trials |
| TP53 | C275G | Different Alteration | Pramlintide (Amylin analogue) | Responsive | THYM | Pre-clinical |
| TP53 | C275G | Different Alteration | AZD6738 (ATR inhibitor) | Responsive | BCL | Early trials |
| TP53 | C141Y | Different Mutation | HSP90 inhibitor | Responsive | CANCER | Pre-clinical |
| TP53 | C141Y | Complete Match | Decitabine (Chemotherapy) | Responsive | AML, MDPS | Early trials |
| TP53 | C141Y | Complete Match | Abemaciclib (CDK4/6 inhibitor) | Resistant | BRCA | Early trials |
| TP53 | C141Y | Different Mutation | HDM2 inhibitor | Responsive | AML | Early trials |
| TP53 | C141Y | Complete Match | Cisplatin (Chemotherapy) | Resistant | FGCT, MGCT | Early trials |
| TP53 | C141Y | Complete Match | AZD6738 (ATR inhibitor) | Responsive | BCL | Early trials |
| TP53 | C141Y | Complete Match | Gemcitabine (Chemotherapy) | Responsive | BLCA | Pre-clinical |
| TP53 | C141Y | Complete Match | Mitomycin C (Chemotherapy) | Responsive | BLCA | Pre-clinical |
| TP53 | C141Y | Complete Match | WEE1 inhibitor | Responsive | HNC | Pre-clinical |
| TP53 | C141Y | Complete Match | MDM2 inhibitor | Resistant | LIP | Early trials |
| TP53 | C141Y | Complete Match | Doxorubicin (Anthracycline antitumor antibiotic) | Responsive | BLCA | Pre-clinical |
| TP53 | C141Y | Complete Match | Pramlintide (Amylin analogue) | Responsive | THYM | Pre-clinical |
| TP53 | C141Y | Complete Match | WEE1 inhibitor | Responsive | OV | Early trials |
| TP53 | C141Y | Different Alteration | Pramlintide (Amylin analogue) | Responsive | THYM | Pre-clinical |
| TP53 | C141Y | Different Alteration | AZD6738 (ATR inhibitor) | Responsive | BCL | Early trials |
| TP53 | W146* | Different Mutation | HSP90 inhibitor | Responsive | CANCER | Pre-clinical |
| TP53 | W146* | Complete Match | Decitabine (Chemotherapy) | Responsive | AML, MDPS | Early trials |
| TP53 | W146* | Complete Match | Abemaciclib (CDK4/6 inhibitor) | Resistant | BRCA | Early trials |
| TP53 | W146* | Different Mutation | HDM2 inhibitor | Responsive | AML | Early trials |
| TP53 | W146* | Complete Match | Cisplatin (Chemotherapy) | Resistant | FGCT, MGCT | Early trials |
| TP53 | W146* | Complete Match | AZD6738 (ATR inhibitor) | Responsive | BCL | Early trials |
| TP53 | W146* | Complete Match | Gemcitabine (Chemotherapy) | Responsive | BLCA | Pre-clinical |
| TP53 | W146* | Complete Match | Mitomycin C (Chemotherapy) | Responsive | BLCA | Pre-clinical |
| TP53 | W146* | Complete Match | WEE1 inhibitor | Responsive | HNC | Pre-clinical |
| TP53 | W146* | Complete Match | MDM2 inhibitor | Resistant | LIP | Early trials |
| TP53 | W146* | Complete Match | Doxorubicin (Anthracycline antitumor antibiotic) | Responsive | BLCA | Pre-clinical |
| TP53 | W146* | Complete Match | Pramlintide (Amylin analogue) | Responsive | THYM | Pre-clinical |
| TP53 | W146* | Complete Match | WEE1 inhibitor | Responsive | OV | Early trials |
| TP53 | W146* | Different Alteration | Pramlintide (Amylin analogue) | Responsive | THYM | Pre-clinical |
| TP53 | W146* | Different Alteration | AZD6738 (ATR inhibitor) | Responsive | BCL | Early trials |
| TP53 | V272M | Different Mutation | HSP90 inhibitor | Responsive | CANCER | Pre-clinical |
| TP53 | V272M | Complete Match | Decitabine (Chemotherapy) | Responsive | AML, MDPS | Early trials |
| TP53 | V272M | Complete Match | Abemaciclib (CDK4/6 inhibitor) | Resistant | BRCA | Early trials |
| TP53 | V272M | Different Mutation | HDM2 inhibitor | Responsive | AML | Early trials |
| TP53 | V272M | Complete Match | Cisplatin (Chemotherapy) | Resistant | FGCT, MGCT | Early trials |
| TP53 | V272M | Complete Match | AZD6738 (ATR inhibitor) | Responsive | BCL | Early trials |
| TP53 | V272M | Complete Match | Gemcitabine (Chemotherapy) | Responsive | BLCA | Pre-clinical |
| TP53 | V272M | Complete Match | Mitomycin C (Chemotherapy) | Responsive | BLCA | Pre-clinical |
| TP53 | V272M | Complete Match | WEE1 inhibitor | Responsive | HNC | Pre-clinical |
| TP53 | V272M | Complete Match | MDM2 inhibitor | Resistant | LIP | Early trials |
| TP53 | V272M | Complete Match | Doxorubicin (Anthracycline antitumor antibiotic) | Responsive | BLCA | Pre-clinical |
| TP53 | V272M | Complete Match | Pramlintide (Amylin analogue) | Responsive | THYM | Pre-clinical |
| TP53 | V272M | Complete Match | WEE1 inhibitor | Responsive | OV | Early trials |
| TP53 | V272M | Different Alteration | Pramlintide (Amylin analogue) | Responsive | THYM | Pre-clinical |
| TP53 | V272M | Different Alteration | AZD6738 (ATR inhibitor) | Responsive | BCL | Early trials |
| TP53 | C242F | Different Mutation | HSP90 inhibitor | Responsive | CANCER | Pre-clinical |
| TP53 | C242F | Complete Match | Decitabine (Chemotherapy) | Responsive | AML, MDPS | Early trials |
| TP53 | C242F | Complete Match | Abemaciclib (CDK4/6 inhibitor) | Resistant | BRCA | Early trials |
| TP53 | C242F | Different Mutation | HDM2 inhibitor | Responsive | AML | Early trials |
| TP53 | C242F | Complete Match | Cisplatin (Chemotherapy) | Resistant | FGCT, MGCT | Early trials |
| TP53 | C242F | Complete Match | AZD6738 (ATR inhibitor) | Responsive | BCL | Early trials |
| TP53 | C242F | Complete Match | Gemcitabine (Chemotherapy) | Responsive | BLCA | Pre-clinical |
| TP53 | C242F | Complete Match | Mitomycin C (Chemotherapy) | Responsive | BLCA | Pre-clinical |
| TP53 | C242F | Complete Match | WEE1 inhibitor | Responsive | HNC | Pre-clinical |
| TP53 | C242F | Complete Match | MDM2 inhibitor | Resistant | LIP | Early trials |
| TP53 | C242F | Complete Match | Doxorubicin (Anthracycline antitumor antibiotic) | Responsive | BLCA | Pre-clinical |
| TP53 | C242F | Complete Match | Pramlintide (Amylin analogue) | Responsive | THYM | Pre-clinical |
| TP53 | C242F | Complete Match | WEE1 inhibitor | Responsive | OV | Early trials |
| TP53 | C242F | Different Alteration | Pramlintide (Amylin analogue) | Responsive | THYM | Pre-clinical |
| TP53 | C242F | Different Alteration | AZD6738 (ATR inhibitor) | Responsive | BCL | Early trials |
| TP53 | P250L | Different Mutation | HSP90 inhibitor | Responsive | CANCER | Pre-clinical |
| TP53 | P250L | Complete Match | Decitabine (Chemotherapy) | Responsive | AML, MDPS | Early trials |
| TP53 | P250L | Complete Match | Abemaciclib (CDK4/6 inhibitor) | Resistant | BRCA | Early trials |
| TP53 | P250L | Different Mutation | HDM2 inhibitor | Responsive | AML | Early trials |
| TP53 | P250L | Complete Match | Cisplatin (Chemotherapy) | Resistant | FGCT, MGCT | Early trials |
| TP53 | P250L | Complete Match | AZD6738 (ATR inhibitor) | Responsive | BCL | Early trials |
| TP53 | P250L | Complete Match | Gemcitabine (Chemotherapy) | Responsive | BLCA | Pre-clinical |
| TP53 | P250L | Complete Match | Mitomycin C (Chemotherapy) | Responsive | BLCA | Pre-clinical |
| TP53 | P250L | Complete Match | WEE1 inhibitor | Responsive | HNC | Pre-clinical |
| TP53 | P250L | Complete Match | MDM2 inhibitor | Resistant | LIP | Early trials |
| TP53 | P250L | Complete Match | Doxorubicin (Anthracycline antitumor antibiotic) | Responsive | BLCA | Pre-clinical |
| TP53 | P250L | Complete Match | Pramlintide (Amylin analogue) | Responsive | THYM | Pre-clinical |
| TP53 | P250L | Complete Match | WEE1 inhibitor | Responsive | OV | Early trials |
| TP53 | P250L | Different Alteration | Pramlintide (Amylin analogue) | Responsive | THYM | Pre-clinical |
| TP53 | P250L | Different Alteration | AZD6738 (ATR inhibitor) | Responsive | BCL | Early trials |
| TP53 | R213* | Different Mutation | HSP90 inhibitor | Responsive | CANCER | Pre-clinical |
| TP53 | R213* | Complete Match | Decitabine (Chemotherapy) | Responsive | AML, MDPS | Early trials |
| TP53 | R213* | Complete Match | Abemaciclib (CDK4/6 inhibitor) | Resistant | BRCA | Early trials |
| TP53 | R213* | Different Mutation | HDM2 inhibitor | Responsive | AML | Early trials |
| TP53 | R213* | Complete Match | Cisplatin (Chemotherapy) | Resistant | FGCT, MGCT | Early trials |
| TP53 | R213* | Complete Match | AZD6738 (ATR inhibitor) | Responsive | BCL | Early trials |
| TP53 | R213* | Complete Match | Gemcitabine (Chemotherapy) | Responsive | BLCA | Pre-clinical |
| TP53 | R213* | Complete Match | Mitomycin C (Chemotherapy) | Responsive | BLCA | Pre-clinical |
| TP53 | R213* | Complete Match | WEE1 inhibitor | Responsive | HNC | Pre-clinical |
| TP53 | R213* | Complete Match | MDM2 inhibitor | Resistant | LIP | Early trials |
| TP53 | R213* | Complete Match | Doxorubicin (Anthracycline antitumor antibiotic) | Responsive | BLCA | Pre-clinical |
| TP53 | R213* | Complete Match | Pramlintide (Amylin analogue) | Responsive | THYM | Pre-clinical |
| TP53 | R213* | Complete Match | WEE1 inhibitor | Responsive | OV | Early trials |
| TP53 | R213* | Different Alteration | Pramlintide (Amylin analogue) | Responsive | THYM | Pre-clinical |
| TP53 | R213* | Different Alteration | AZD6738 (ATR inhibitor) | Responsive | BCL | Early trials |
| TP53 | P278L | Different Mutation | HSP90 inhibitor | Responsive | CANCER | Pre-clinical |
| TP53 | P278L | Complete Match | Decitabine (Chemotherapy) | Responsive | AML, MDPS | Early trials |
| TP53 | P278L | Complete Match | Abemaciclib (CDK4/6 inhibitor) | Resistant | BRCA | Early trials |
| TP53 | P278L | Different Mutation | HDM2 inhibitor | Responsive | AML | Early trials |
| TP53 | P278L | Complete Match | Cisplatin (Chemotherapy) | Resistant | FGCT, MGCT | Early trials |
| TP53 | P278L | Complete Match | AZD6738 (ATR inhibitor) | Responsive | BCL | Early trials |
| TP53 | P278L | Complete Match | Gemcitabine (Chemotherapy) | Responsive | BLCA | Pre-clinical |
| TP53 | P278L | Complete Match | Mitomycin C (Chemotherapy) | Responsive | BLCA | Pre-clinical |
| TP53 | P278L | Complete Match | WEE1 inhibitor | Responsive | HNC | Pre-clinical |
| TP53 | P278L | Complete Match | MDM2 inhibitor | Resistant | LIP | Early trials |
| TP53 | P278L | Complete Match | Doxorubicin (Anthracycline antitumor antibiotic) | Responsive | BLCA | Pre-clinical |
| TP53 | P278L | Complete Match | Pramlintide (Amylin analogue) | Responsive | THYM | Pre-clinical |
| TP53 | P278L | Complete Match | WEE1 inhibitor | Responsive | OV | Early trials |
| TP53 | P278L | Different Alteration | Pramlintide (Amylin analogue) | Responsive | THYM | Pre-clinical |
| TP53 | P278L | Different Alteration | AZD6738 (ATR inhibitor) | Responsive | BCL | Early trials |
| TP53 | P151S | Different Mutation | HSP90 inhibitor | Responsive | CANCER | Pre-clinical |
| TP53 | P151S | Complete Match | Decitabine (Chemotherapy) | Responsive | AML, MDPS | Early trials |
| TP53 | P151S | Complete Match | Abemaciclib (CDK4/6 inhibitor) | Resistant | BRCA | Early trials |
| TP53 | P151S | Different Mutation | HDM2 inhibitor | Responsive | AML | Early trials |
| TP53 | P151S | Complete Match | Cisplatin (Chemotherapy) | Resistant | FGCT, MGCT | Early trials |
| TP53 | P151S | Complete Match | AZD6738 (ATR inhibitor) | Responsive | BCL | Early trials |
| TP53 | P151S | Complete Match | Gemcitabine (Chemotherapy) | Responsive | BLCA | Pre-clinical |
| TP53 | P151S | Complete Match | Mitomycin C (Chemotherapy) | Responsive | BLCA | Pre-clinical |
| TP53 | P151S | Complete Match | WEE1 inhibitor | Responsive | HNC | Pre-clinical |
| TP53 | P151S | Complete Match | MDM2 inhibitor | Resistant | LIP | Early trials |
| TP53 | P151S | Complete Match | Doxorubicin (Anthracycline antitumor antibiotic) | Responsive | BLCA | Pre-clinical |
| TP53 | P151S | Complete Match | Pramlintide (Amylin analogue) | Responsive | THYM | Pre-clinical |
| TP53 | P151S | Complete Match | WEE1 inhibitor | Responsive | OV | Early trials |
| TP53 | P151S | Different Alteration | Pramlintide (Amylin analogue) | Responsive | THYM | Pre-clinical |
| TP53 | P151S | Different Alteration | AZD6738 (ATR inhibitor) | Responsive | BCL | Early trials |
| TP53 | V173L | Different Mutation | HSP90 inhibitor | Responsive | CANCER | Pre-clinical |
| TP53 | V173L | Complete Match | Decitabine (Chemotherapy) | Responsive | AML, MDPS | Early trials |
| TP53 | V173L | Complete Match | Abemaciclib (CDK4/6 inhibitor) | Resistant | BRCA | Early trials |
| TP53 | V173L | Different Mutation | HDM2 inhibitor | Responsive | AML | Early trials |
| TP53 | V173L | Complete Match | Cisplatin (Chemotherapy) | Resistant | FGCT, MGCT | Early trials |
| TP53 | V173L | Complete Match | AZD6738 (ATR inhibitor) | Responsive | BCL | Early trials |
| TP53 | V173L | Complete Match | Gemcitabine (Chemotherapy) | Responsive | BLCA | Pre-clinical |
| TP53 | V173L | Complete Match | Mitomycin C (Chemotherapy) | Responsive | BLCA | Pre-clinical |
| TP53 | V173L | Complete Match | WEE1 inhibitor | Responsive | HNC | Pre-clinical |
| TP53 | V173L | Complete Match | MDM2 inhibitor | Resistant | LIP | Early trials |
| TP53 | V173L | Complete Match | Doxorubicin (Anthracycline antitumor antibiotic) | Responsive | BLCA | Pre-clinical |
| TP53 | V173L | Complete Match | Pramlintide (Amylin analogue) | Responsive | THYM | Pre-clinical |
| TP53 | V173L | Complete Match | WEE1 inhibitor | Responsive | OV | Early trials |
| TP53 | V173L | Different Alteration | Pramlintide (Amylin analogue) | Responsive | THYM | Pre-clinical |
| TP53 | V173L | Different Alteration | AZD6738 (ATR inhibitor) | Responsive | BCL | Early trials |
| TP53 | L257P | Different Mutation | HSP90 inhibitor | Responsive | CANCER | Pre-clinical |
| TP53 | L257P | Complete Match | Decitabine (Chemotherapy) | Responsive | AML, MDPS | Early trials |
| TP53 | L257P | Complete Match | Abemaciclib (CDK4/6 inhibitor) | Resistant | BRCA | Early trials |
| TP53 | L257P | Different Mutation | HDM2 inhibitor | Responsive | AML | Early trials |
| TP53 | L257P | Complete Match | Cisplatin (Chemotherapy) | Resistant | FGCT, MGCT | Early trials |
| TP53 | L257P | Complete Match | AZD6738 (ATR inhibitor) | Responsive | BCL | Early trials |
| TP53 | L257P | Complete Match | Gemcitabine (Chemotherapy) | Responsive | BLCA | Pre-clinical |
| TP53 | L257P | Complete Match | Mitomycin C (Chemotherapy) | Responsive | BLCA | Pre-clinical |
| TP53 | L257P | Complete Match | WEE1 inhibitor | Responsive | HNC | Pre-clinical |
| TP53 | L257P | Complete Match | MDM2 inhibitor | Resistant | LIP | Early trials |
| TP53 | L257P | Complete Match | Doxorubicin (Anthracycline antitumor antibiotic) | Responsive | BLCA | Pre-clinical |
| TP53 | L257P | Complete Match | Pramlintide (Amylin analogue) | Responsive | THYM | Pre-clinical |
| TP53 | L257P | Complete Match | WEE1 inhibitor | Responsive | OV | Early trials |
| TP53 | L257P | Different Alteration | Pramlintide (Amylin analogue) | Responsive | THYM | Pre-clinical |
| TP53 | L257P | Different Alteration | AZD6738 (ATR inhibitor) | Responsive | BCL | Early trials |
| TP53 | R181H), TP53 MUT (R342* | Different Mutation | HSP90 inhibitor | Responsive | CANCER | Pre-clinical |
| TP53 | R181H), TP53 MUT (R342* | Complete Match | Decitabine (Chemotherapy) | Responsive | AML, MDPS | Early trials |
| TP53 | R181H), TP53 MUT (R342* | Complete Match | Abemaciclib (CDK4/6 inhibitor) | Resistant | BRCA | Early trials |
| TP53 | R181H), TP53 MUT (R342* | Different Mutation | HDM2 inhibitor | Responsive | AML | Early trials |
| TP53 | R181H), TP53 MUT (R342* | Complete Match | Cisplatin (Chemotherapy) | Resistant | FGCT, MGCT | Early trials |
| TP53 | R181H), TP53 MUT (R342* | Complete Match | AZD6738 (ATR inhibitor) | Responsive | BCL | Early trials |
| TP53 | R181H), TP53 MUT (R342* | Complete Match | Gemcitabine (Chemotherapy) | Responsive | BLCA | Pre-clinical |
| TP53 | R181H), TP53 MUT (R342* | Complete Match | Mitomycin C (Chemotherapy) | Responsive | BLCA | Pre-clinical |
| TP53 | R181H), TP53 MUT (R342* | Complete Match | WEE1 inhibitor | Responsive | HNC | Pre-clinical |
| TP53 | R181H), TP53 MUT (R342* | Complete Match | MDM2 inhibitor | Resistant | LIP | Early trials |
| TP53 | R181H), TP53 MUT (R342* | Complete Match | Doxorubicin (Anthracycline antitumor antibiotic) | Responsive | BLCA | Pre-clinical |
| TP53 | R181H), TP53 MUT (R342* | Complete Match | Pramlintide (Amylin analogue) | Responsive | THYM | Pre-clinical |
| TP53 | R181H), TP53 MUT (R342* | Complete Match | WEE1 inhibitor | Responsive | OV | Early trials |
| TP53 | R181H), TP53 MUT (R342* | Different Alteration | Pramlintide (Amylin analogue) | Responsive | THYM | Pre-clinical |
| TP53 | R181H), TP53 MUT (R342* | Different Alteration | AZD6738 (ATR inhibitor) | Responsive | BCL | Early trials |
| TP53 | C242S | Different Mutation | HSP90 inhibitor | Responsive | CANCER | Pre-clinical |
| TP53 | C242S | Complete Match | Decitabine (Chemotherapy) | Responsive | AML, MDPS | Early trials |
| TP53 | C242S | Complete Match | Abemaciclib (CDK4/6 inhibitor) | Resistant | BRCA | Early trials |
| TP53 | C242S | Different Mutation | HDM2 inhibitor | Responsive | AML | Early trials |
| TP53 | C242S | Complete Match | Cisplatin (Chemotherapy) | Resistant | FGCT, MGCT | Early trials |
| TP53 | C242S | Complete Match | AZD6738 (ATR inhibitor) | Responsive | BCL | Early trials |
| TP53 | C242S | Complete Match | Gemcitabine (Chemotherapy) | Responsive | BLCA | Pre-clinical |
| TP53 | C242S | Complete Match | Mitomycin C (Chemotherapy) | Responsive | BLCA | Pre-clinical |
| TP53 | C242S | Complete Match | WEE1 inhibitor | Responsive | HNC | Pre-clinical |
| TP53 | C242S | Complete Match | MDM2 inhibitor | Resistant | LIP | Early trials |
| TP53 | C242S | Complete Match | Doxorubicin (Anthracycline antitumor antibiotic) | Responsive | BLCA | Pre-clinical |
| TP53 | C242S | Complete Match | Pramlintide (Amylin analogue) | Responsive | THYM | Pre-clinical |
| TP53 | C242S | Complete Match | WEE1 inhibitor | Responsive | OV | Early trials |
| TP53 | C242S | Different Alteration | Pramlintide (Amylin analogue) | Responsive | THYM | Pre-clinical |
| TP53 | C242S | Different Alteration | AZD6738 (ATR inhibitor) | Responsive | BCL | Early trials |
| TP53 | R337C | Different Mutation | HSP90 inhibitor | Responsive | CANCER | Pre-clinical |
| TP53 | R337C | Complete Match | Decitabine (Chemotherapy) | Responsive | AML, MDPS | Early trials |
| TP53 | R337C | Complete Match | Abemaciclib (CDK4/6 inhibitor) | Resistant | BRCA | Early trials |
| TP53 | R337C | Different Mutation | HDM2 inhibitor | Responsive | AML | Early trials |
| TP53 | R337C | Complete Match | Cisplatin (Chemotherapy) | Resistant | FGCT, MGCT | Early trials |
| TP53 | R337C | Complete Match | AZD6738 (ATR inhibitor) | Responsive | BCL | Early trials |
| TP53 | R337C | Complete Match | Gemcitabine (Chemotherapy) | Responsive | BLCA | Pre-clinical |
| TP53 | R337C | Complete Match | Mitomycin C (Chemotherapy) | Responsive | BLCA | Pre-clinical |
| TP53 | R337C | Complete Match | WEE1 inhibitor | Responsive | HNC | Pre-clinical |
| TP53 | R337C | Complete Match | MDM2 inhibitor | Resistant | LIP | Early trials |
| TP53 | R337C | Complete Match | Doxorubicin (Anthracycline antitumor antibiotic) | Responsive | BLCA | Pre-clinical |
| TP53 | R337C | Complete Match | Pramlintide (Amylin analogue) | Responsive | THYM | Pre-clinical |
| TP53 | R337C | Complete Match | WEE1 inhibitor | Responsive | OV | Early trials |
| TP53 | R337C | Different Alteration | Pramlintide (Amylin analogue) | Responsive | THYM | Pre-clinical |
| TP53 | R337C | Different Alteration | AZD6738 (ATR inhibitor) | Responsive | BCL | Early trials |
| TP53 | G245D | Different Mutation | HSP90 inhibitor | Responsive | CANCER | Pre-clinical |
| TP53 | G245D | Complete Match | Decitabine (Chemotherapy) | Responsive | AML, MDPS | Early trials |
| TP53 | G245D | Complete Match | Abemaciclib (CDK4/6 inhibitor) | Resistant | BRCA | Early trials |
| TP53 | G245D | Different Mutation | HDM2 inhibitor | Responsive | AML | Early trials |
| TP53 | G245D | Complete Match | Cisplatin (Chemotherapy) | Resistant | FGCT, MGCT | Early trials |
| TP53 | G245D | Complete Match | AZD6738 (ATR inhibitor) | Responsive | BCL | Early trials |
| TP53 | G245D | Complete Match | Gemcitabine (Chemotherapy) | Responsive | BLCA | Pre-clinical |
| TP53 | G245D | Complete Match | Mitomycin C (Chemotherapy) | Responsive | BLCA | Pre-clinical |
| TP53 | G245D | Complete Match | WEE1 inhibitor | Responsive | HNC | Pre-clinical |
| TP53 | G245D | Complete Match | MDM2 inhibitor | Resistant | LIP | Early trials |
| TP53 | G245D | Complete Match | Doxorubicin (Anthracycline antitumor antibiotic) | Responsive | BLCA | Pre-clinical |
| TP53 | G245D | Complete Match | Pramlintide (Amylin analogue) | Responsive | THYM | Pre-clinical |
| TP53 | G245D | Complete Match | WEE1 inhibitor | Responsive | OV | Early trials |
| TP53 | G245D | Different Alteration | Pramlintide (Amylin analogue) | Responsive | THYM | Pre-clinical |
| TP53 | G245D | Different Alteration | AZD6738 (ATR inhibitor) | Responsive | BCL | Early trials |
| TP53 | C135W | Different Mutation | HSP90 inhibitor | Responsive | CANCER | Pre-clinical |
| TP53 | C135W | Complete Match | Decitabine (Chemotherapy) | Responsive | AML, MDPS | Early trials |
| TP53 | C135W | Complete Match | Abemaciclib (CDK4/6 inhibitor) | Resistant | BRCA | Early trials |
| TP53 | C135W | Different Mutation | HDM2 inhibitor | Responsive | AML | Early trials |
| TP53 | C135W | Complete Match | Cisplatin (Chemotherapy) | Resistant | FGCT, MGCT | Early trials |
| TP53 | C135W | Complete Match | AZD6738 (ATR inhibitor) | Responsive | BCL | Early trials |
| TP53 | C135W | Complete Match | Gemcitabine (Chemotherapy) | Responsive | BLCA | Pre-clinical |
| TP53 | C135W | Complete Match | Mitomycin C (Chemotherapy) | Responsive | BLCA | Pre-clinical |
| TP53 | C135W | Complete Match | WEE1 inhibitor | Responsive | HNC | Pre-clinical |
| TP53 | C135W | Complete Match | MDM2 inhibitor | Resistant | LIP | Early trials |
| TP53 | C135W | Complete Match | Doxorubicin (Anthracycline antitumor antibiotic) | Responsive | BLCA | Pre-clinical |
| TP53 | C135W | Complete Match | Pramlintide (Amylin analogue) | Responsive | THYM | Pre-clinical |
| TP53 | C135W | Complete Match | WEE1 inhibitor | Responsive | OV | Early trials |
| TP53 | C135W | Different Alteration | Pramlintide (Amylin analogue) | Responsive | THYM | Pre-clinical |
| TP53 | C135W | Different Alteration | AZD6738 (ATR inhibitor) | Responsive | BCL | Early trials |
| TP53 | S241F | Different Mutation | HSP90 inhibitor | Responsive | CANCER | Pre-clinical |
| TP53 | S241F | Complete Match | Decitabine (Chemotherapy) | Responsive | AML, MDPS | Early trials |
| TP53 | S241F | Complete Match | Abemaciclib (CDK4/6 inhibitor) | Resistant | BRCA | Early trials |
| TP53 | S241F | Different Mutation | HDM2 inhibitor | Responsive | AML | Early trials |
| TP53 | S241F | Complete Match | Cisplatin (Chemotherapy) | Resistant | FGCT, MGCT | Early trials |
| TP53 | S241F | Complete Match | AZD6738 (ATR inhibitor) | Responsive | BCL | Early trials |
| TP53 | S241F | Complete Match | Gemcitabine (Chemotherapy) | Responsive | BLCA | Pre-clinical |
| TP53 | S241F | Complete Match | Mitomycin C (Chemotherapy) | Responsive | BLCA | Pre-clinical |
| TP53 | S241F | Complete Match | WEE1 inhibitor | Responsive | HNC | Pre-clinical |
| TP53 | S241F | Complete Match | MDM2 inhibitor | Resistant | LIP | Early trials |
| TP53 | S241F | Complete Match | Doxorubicin (Anthracycline antitumor antibiotic) | Responsive | BLCA | Pre-clinical |
| TP53 | S241F | Complete Match | Pramlintide (Amylin analogue) | Responsive | THYM | Pre-clinical |
| TP53 | S241F | Complete Match | WEE1 inhibitor | Responsive | OV | Early trials |
| TP53 | S241F | Different Alteration | Pramlintide (Amylin analogue) | Responsive | THYM | Pre-clinical |
| TP53 | S241F | Different Alteration | AZD6738 (ATR inhibitor) | Responsive | BCL | Early trials |
| TP53 | Y205H | Different Mutation | HSP90 inhibitor | Responsive | CANCER | Pre-clinical |
| TP53 | Y205H | Complete Match | Decitabine (Chemotherapy) | Responsive | AML, MDPS | Early trials |
| TP53 | Y205H | Complete Match | Abemaciclib (CDK4/6 inhibitor) | Resistant | BRCA | Early trials |
| TP53 | Y205H | Different Mutation | HDM2 inhibitor | Responsive | AML | Early trials |
| TP53 | Y205H | Complete Match | Cisplatin (Chemotherapy) | Resistant | FGCT, MGCT | Early trials |
| TP53 | Y205H | Complete Match | AZD6738 (ATR inhibitor) | Responsive | BCL | Early trials |
| TP53 | Y205H | Complete Match | Gemcitabine (Chemotherapy) | Responsive | BLCA | Pre-clinical |
| TP53 | Y205H | Complete Match | Mitomycin C (Chemotherapy) | Responsive | BLCA | Pre-clinical |
| TP53 | Y205H | Complete Match | WEE1 inhibitor | Responsive | HNC | Pre-clinical |
| TP53 | Y205H | Complete Match | MDM2 inhibitor | Resistant | LIP | Early trials |
| TP53 | Y205H | Complete Match | Doxorubicin (Anthracycline antitumor antibiotic) | Responsive | BLCA | Pre-clinical |
| TP53 | Y205H | Complete Match | Pramlintide (Amylin analogue) | Responsive | THYM | Pre-clinical |
| TP53 | Y205H | Complete Match | WEE1 inhibitor | Responsive | OV | Early trials |
| TP53 | Y205H | Different Alteration | Pramlintide (Amylin analogue) | Responsive | THYM | Pre-clinical |
| TP53 | Y205H | Different Alteration | AZD6738 (ATR inhibitor) | Responsive | BCL | Early trials |
| TP53 | C176W | Different Mutation | HSP90 inhibitor | Responsive | CANCER | Pre-clinical |
| TP53 | C176W | Complete Match | Decitabine (Chemotherapy) | Responsive | AML, MDPS | Early trials |
| TP53 | C176W | Complete Match | Abemaciclib (CDK4/6 inhibitor) | Resistant | BRCA | Early trials |
| TP53 | C176W | Different Mutation | HDM2 inhibitor | Responsive | AML | Early trials |
| TP53 | C176W | Complete Match | Cisplatin (Chemotherapy) | Resistant | FGCT, MGCT | Early trials |
| TP53 | C176W | Complete Match | AZD6738 (ATR inhibitor) | Responsive | BCL | Early trials |
| TP53 | C176W | Complete Match | Gemcitabine (Chemotherapy) | Responsive | BLCA | Pre-clinical |
| TP53 | C176W | Complete Match | Mitomycin C (Chemotherapy) | Responsive | BLCA | Pre-clinical |
| TP53 | C176W | Complete Match | WEE1 inhibitor | Responsive | HNC | Pre-clinical |
| TP53 | C176W | Complete Match | MDM2 inhibitor | Resistant | LIP | Early trials |
| TP53 | C176W | Complete Match | Doxorubicin (Anthracycline antitumor antibiotic) | Responsive | BLCA | Pre-clinical |
| TP53 | C176W | Complete Match | Pramlintide (Amylin analogue) | Responsive | THYM | Pre-clinical |
| TP53 | C176W | Complete Match | WEE1 inhibitor | Responsive | OV | Early trials |
| TP53 | C176W | Different Alteration | Pramlintide (Amylin analogue) | Responsive | THYM | Pre-clinical |
| TP53 | C176W | Different Alteration | AZD6738 (ATR inhibitor) | Responsive | BCL | Early trials |
| TP53 | E294* | Different Mutation | HSP90 inhibitor | Responsive | CANCER | Pre-clinical |
| TP53 | E294* | Complete Match | Decitabine (Chemotherapy) | Responsive | AML, MDPS | Early trials |
| TP53 | E294* | Complete Match | Abemaciclib (CDK4/6 inhibitor) | Resistant | BRCA | Early trials |
| TP53 | E294* | Different Mutation | HDM2 inhibitor | Responsive | AML | Early trials |
| TP53 | E294* | Complete Match | Cisplatin (Chemotherapy) | Resistant | FGCT, MGCT | Early trials |
| TP53 | E294* | Complete Match | AZD6738 (ATR inhibitor) | Responsive | BCL | Early trials |
| TP53 | E294* | Complete Match | Gemcitabine (Chemotherapy) | Responsive | BLCA | Pre-clinical |
| TP53 | E294* | Complete Match | Mitomycin C (Chemotherapy) | Responsive | BLCA | Pre-clinical |
| TP53 | E294* | Complete Match | WEE1 inhibitor | Responsive | HNC | Pre-clinical |
| TP53 | E294* | Complete Match | MDM2 inhibitor | Resistant | LIP | Early trials |
| TP53 | E294* | Complete Match | Doxorubicin (Anthracycline antitumor antibiotic) | Responsive | BLCA | Pre-clinical |
| TP53 | E294* | Complete Match | Pramlintide (Amylin analogue) | Responsive | THYM | Pre-clinical |
| TP53 | E294* | Complete Match | WEE1 inhibitor | Responsive | OV | Early trials |
| TP53 | E294* | Different Alteration | Pramlintide (Amylin analogue) | Responsive | THYM | Pre-clinical |
| TP53 | E294* | Different Alteration | AZD6738 (ATR inhibitor) | Responsive | BCL | Early trials |
| TP53 | Y220C | Different Mutation | HSP90 inhibitor | Responsive | CANCER | Pre-clinical |
| TP53 | Y220C | Complete Match | Decitabine (Chemotherapy) | Responsive | AML, MDPS | Early trials |
| TP53 | Y220C | Complete Match | Abemaciclib (CDK4/6 inhibitor) | Resistant | BRCA | Early trials |
| TP53 | Y220C | Different Mutation | HDM2 inhibitor | Responsive | AML | Early trials |
| TP53 | Y220C | Complete Match | Cisplatin (Chemotherapy) | Resistant | FGCT, MGCT | Early trials |
| TP53 | Y220C | Complete Match | AZD6738 (ATR inhibitor) | Responsive | BCL | Early trials |
| TP53 | Y220C | Complete Match | Gemcitabine (Chemotherapy) | Responsive | BLCA | Pre-clinical |
| TP53 | Y220C | Complete Match | Mitomycin C (Chemotherapy) | Responsive | BLCA | Pre-clinical |
| TP53 | Y220C | Complete Match | WEE1 inhibitor | Responsive | HNC | Pre-clinical |
| TP53 | Y220C | Complete Match | MDM2 inhibitor | Resistant | LIP | Early trials |
| TP53 | Y220C | Complete Match | Doxorubicin (Anthracycline antitumor antibiotic) | Responsive | BLCA | Pre-clinical |
| TP53 | Y220C | Complete Match | Pramlintide (Amylin analogue) | Responsive | THYM | Pre-clinical |
| TP53 | Y220C | Complete Match | WEE1 inhibitor | Responsive | OV | Early trials |
| TP53 | Y220C | Different Alteration | Pramlintide (Amylin analogue) | Responsive | THYM | Pre-clinical |
| TP53 | Y220C | Different Alteration | AZD6738 (ATR inhibitor) | Responsive | BCL | Early trials |
| TP53 | D281N | Different Mutation | HSP90 inhibitor | Responsive | CANCER | Pre-clinical |
| TP53 | D281N | Complete Match | Decitabine (Chemotherapy) | Responsive | AML, MDPS | Early trials |
| TP53 | D281N | Complete Match | Abemaciclib (CDK4/6 inhibitor) | Resistant | BRCA | Early trials |
| TP53 | D281N | Different Mutation | HDM2 inhibitor | Responsive | AML | Early trials |
| TP53 | D281N | Complete Match | Cisplatin (Chemotherapy) | Resistant | FGCT, MGCT | Early trials |
| TP53 | D281N | Complete Match | AZD6738 (ATR inhibitor) | Responsive | BCL | Early trials |
| TP53 | D281N | Complete Match | Gemcitabine (Chemotherapy) | Responsive | BLCA | Pre-clinical |
| TP53 | D281N | Complete Match | Mitomycin C (Chemotherapy) | Responsive | BLCA | Pre-clinical |
| TP53 | D281N | Complete Match | WEE1 inhibitor | Responsive | HNC | Pre-clinical |
| TP53 | D281N | Complete Match | MDM2 inhibitor | Resistant | LIP | Early trials |
| TP53 | D281N | Complete Match | Doxorubicin (Anthracycline antitumor antibiotic) | Responsive | BLCA | Pre-clinical |
| TP53 | D281N | Complete Match | Pramlintide (Amylin analogue) | Responsive | THYM | Pre-clinical |
| TP53 | D281N | Complete Match | WEE1 inhibitor | Responsive | OV | Early trials |
| TP53 | D281N | Different Alteration | Pramlintide (Amylin analogue) | Responsive | THYM | Pre-clinical |
| TP53 | D281N | Different Alteration | AZD6738 (ATR inhibitor) | Responsive | BCL | Early trials |
| TP53 | V216L | Different Mutation | HSP90 inhibitor | Responsive | CANCER | Pre-clinical |
| TP53 | V216L | Complete Match | Decitabine (Chemotherapy) | Responsive | AML, MDPS | Early trials |
| TP53 | V216L | Complete Match | Abemaciclib (CDK4/6 inhibitor) | Resistant | BRCA | Early trials |
| TP53 | V216L | Different Mutation | HDM2 inhibitor | Responsive | AML | Early trials |
| TP53 | V216L | Complete Match | Cisplatin (Chemotherapy) | Resistant | FGCT, MGCT | Early trials |
| TP53 | V216L | Complete Match | AZD6738 (ATR inhibitor) | Responsive | BCL | Early trials |
| TP53 | V216L | Complete Match | Gemcitabine (Chemotherapy) | Responsive | BLCA | Pre-clinical |
| TP53 | V216L | Complete Match | Mitomycin C (Chemotherapy) | Responsive | BLCA | Pre-clinical |
| TP53 | V216L | Complete Match | WEE1 inhibitor | Responsive | HNC | Pre-clinical |
| TP53 | V216L | Complete Match | MDM2 inhibitor | Resistant | LIP | Early trials |
| TP53 | V216L | Complete Match | Doxorubicin (Anthracycline antitumor antibiotic) | Responsive | BLCA | Pre-clinical |
| TP53 | V216L | Complete Match | Pramlintide (Amylin analogue) | Responsive | THYM | Pre-clinical |
| TP53 | V216L | Complete Match | WEE1 inhibitor | Responsive | OV | Early trials |
| TP53 | V216L | Different Alteration | Pramlintide (Amylin analogue) | Responsive | THYM | Pre-clinical |
| TP53 | V216L | Different Alteration | AZD6738 (ATR inhibitor) | Responsive | BCL | Early trials |
| TP53 | R158P | Different Mutation | HSP90 inhibitor | Responsive | CANCER | Pre-clinical |
| TP53 | R158P | Complete Match | Decitabine (Chemotherapy) | Responsive | AML, MDPS | Early trials |
| TP53 | R158P | Complete Match | Abemaciclib (CDK4/6 inhibitor) | Resistant | BRCA | Early trials |
| TP53 | R158P | Different Mutation | HDM2 inhibitor | Responsive | AML | Early trials |
| TP53 | R158P | Complete Match | Cisplatin (Chemotherapy) | Resistant | FGCT, MGCT | Early trials |
| TP53 | R158P | Complete Match | AZD6738 (ATR inhibitor) | Responsive | BCL | Early trials |
| TP53 | R158P | Complete Match | Gemcitabine (Chemotherapy) | Responsive | BLCA | Pre-clinical |
| TP53 | R158P | Complete Match | Mitomycin C (Chemotherapy) | Responsive | BLCA | Pre-clinical |
| TP53 | R158P | Complete Match | WEE1 inhibitor | Responsive | HNC | Pre-clinical |
| TP53 | R158P | Complete Match | MDM2 inhibitor | Resistant | LIP | Early trials |
| TP53 | R158P | Complete Match | Doxorubicin (Anthracycline antitumor antibiotic) | Responsive | BLCA | Pre-clinical |
| TP53 | R158P | Complete Match | Pramlintide (Amylin analogue) | Responsive | THYM | Pre-clinical |
| TP53 | R158P | Complete Match | WEE1 inhibitor | Responsive | OV | Early trials |
| TP53 | R158P | Different Alteration | Pramlintide (Amylin analogue) | Responsive | THYM | Pre-clinical |
| TP53 | R158P | Different Alteration | AZD6738 (ATR inhibitor) | Responsive | BCL | Early trials |
| TP53 | splice_donor_variant), TP53 MUT (splice_donor_variant | Different Mutation | HSP90 inhibitor | Responsive | CANCER | Pre-clinical |
| TP53 | splice_donor_variant), TP53 MUT (splice_donor_variant | Complete Match | Decitabine (Chemotherapy) | Responsive | AML, MDPS | Early trials |
| TP53 | splice_donor_variant), TP53 MUT (splice_donor_variant | Complete Match | Abemaciclib (CDK4/6 inhibitor) | Resistant | BRCA | Early trials |
| TP53 | splice_donor_variant), TP53 MUT (splice_donor_variant | Different Mutation | HDM2 inhibitor | Responsive | AML | Early trials |
| TP53 | splice_donor_variant), TP53 MUT (splice_donor_variant | Complete Match | Cisplatin (Chemotherapy) | Resistant | FGCT, MGCT | Early trials |
| TP53 | splice_donor_variant), TP53 MUT (splice_donor_variant | Complete Match | AZD6738 (ATR inhibitor) | Responsive | BCL | Early trials |
| TP53 | splice_donor_variant), TP53 MUT (splice_donor_variant | Complete Match | Gemcitabine (Chemotherapy) | Responsive | BLCA | Pre-clinical |
| TP53 | splice_donor_variant), TP53 MUT (splice_donor_variant | Complete Match | Mitomycin C (Chemotherapy) | Responsive | BLCA | Pre-clinical |
| TP53 | splice_donor_variant), TP53 MUT (splice_donor_variant | Complete Match | WEE1 inhibitor | Responsive | HNC | Pre-clinical |
| TP53 | splice_donor_variant), TP53 MUT (splice_donor_variant | Complete Match | MDM2 inhibitor | Resistant | LIP | Early trials |
| TP53 | splice_donor_variant), TP53 MUT (splice_donor_variant | Complete Match | Doxorubicin (Anthracycline antitumor antibiotic) | Responsive | BLCA | Pre-clinical |
| TP53 | splice_donor_variant), TP53 MUT (splice_donor_variant | Complete Match | Pramlintide (Amylin analogue) | Responsive | THYM | Pre-clinical |
| TP53 | splice_donor_variant), TP53 MUT (splice_donor_variant | Complete Match | WEE1 inhibitor | Responsive | OV | Early trials |
| TP53 | splice_donor_variant), TP53 MUT (splice_donor_variant | Different Alteration | Pramlintide (Amylin analogue) | Responsive | THYM | Pre-clinical |
| TP53 | splice_donor_variant), TP53 MUT (splice_donor_variant | Different Alteration | AZD6738 (ATR inhibitor) | Responsive | BCL | Early trials |
| TP53 | H179R | Different Mutation | HSP90 inhibitor | Responsive | CANCER | Pre-clinical |
| TP53 | H179R | Complete Match | Decitabine (Chemotherapy) | Responsive | AML, MDPS | Early trials |
| TP53 | H179R | Complete Match | Abemaciclib (CDK4/6 inhibitor) | Resistant | BRCA | Early trials |
| TP53 | H179R | Different Mutation | HDM2 inhibitor | Responsive | AML | Early trials |
| TP53 | H179R | Complete Match | Cisplatin (Chemotherapy) | Resistant | FGCT, MGCT | Early trials |
| TP53 | H179R | Complete Match | AZD6738 (ATR inhibitor) | Responsive | BCL | Early trials |
| TP53 | H179R | Complete Match | Gemcitabine (Chemotherapy) | Responsive | BLCA | Pre-clinical |
| TP53 | H179R | Complete Match | Mitomycin C (Chemotherapy) | Responsive | BLCA | Pre-clinical |
| TP53 | H179R | Complete Match | WEE1 inhibitor | Responsive | HNC | Pre-clinical |
| TP53 | H179R | Complete Match | MDM2 inhibitor | Resistant | LIP | Early trials |
| TP53 | H179R | Complete Match | Doxorubicin (Anthracycline antitumor antibiotic) | Responsive | BLCA | Pre-clinical |
| TP53 | H179R | Complete Match | Pramlintide (Amylin analogue) | Responsive | THYM | Pre-clinical |
| TP53 | H179R | Complete Match | WEE1 inhibitor | Responsive | OV | Early trials |
| TP53 | H179R | Different Alteration | Pramlintide (Amylin analogue) | Responsive | THYM | Pre-clinical |
| TP53 | H179R | Different Alteration | AZD6738 (ATR inhibitor) | Responsive | BCL | Early trials |
| TP53 | Q104* | Different Mutation | HSP90 inhibitor | Responsive | CANCER | Pre-clinical |
| TP53 | Q104* | Complete Match | Decitabine (Chemotherapy) | Responsive | AML, MDPS | Early trials |
| TP53 | Q104* | Complete Match | Abemaciclib (CDK4/6 inhibitor) | Resistant | BRCA | Early trials |
| TP53 | Q104* | Different Mutation | HDM2 inhibitor | Responsive | AML | Early trials |
| TP53 | Q104* | Complete Match | Cisplatin (Chemotherapy) | Resistant | FGCT, MGCT | Early trials |
| TP53 | Q104* | Complete Match | AZD6738 (ATR inhibitor) | Responsive | BCL | Early trials |
| TP53 | Q104* | Complete Match | Gemcitabine (Chemotherapy) | Responsive | BLCA | Pre-clinical |
| TP53 | Q104* | Complete Match | Mitomycin C (Chemotherapy) | Responsive | BLCA | Pre-clinical |
| TP53 | Q104* | Complete Match | WEE1 inhibitor | Responsive | HNC | Pre-clinical |
| TP53 | Q104* | Complete Match | MDM2 inhibitor | Resistant | LIP | Early trials |
| TP53 | Q104* | Complete Match | Doxorubicin (Anthracycline antitumor antibiotic) | Responsive | BLCA | Pre-clinical |
| TP53 | Q104* | Complete Match | Pramlintide (Amylin analogue) | Responsive | THYM | Pre-clinical |
| TP53 | Q104* | Complete Match | WEE1 inhibitor | Responsive | OV | Early trials |
| TP53 | Q104* | Different Alteration | Pramlintide (Amylin analogue) | Responsive | THYM | Pre-clinical |
| TP53 | Q104* | Different Alteration | AZD6738 (ATR inhibitor) | Responsive | BCL | Early trials |
| TP53 | N268I | Different Mutation | HSP90 inhibitor | Responsive | CANCER | Pre-clinical |
| TP53 | N268I | Complete Match | Decitabine (Chemotherapy) | Responsive | AML, MDPS | Early trials |
| TP53 | N268I | Complete Match | Abemaciclib (CDK4/6 inhibitor) | Resistant | BRCA | Early trials |
| TP53 | N268I | Different Mutation | HDM2 inhibitor | Responsive | AML | Early trials |
| TP53 | N268I | Complete Match | Cisplatin (Chemotherapy) | Resistant | FGCT, MGCT | Early trials |
| TP53 | N268I | Complete Match | AZD6738 (ATR inhibitor) | Responsive | BCL | Early trials |
| TP53 | N268I | Complete Match | Gemcitabine (Chemotherapy) | Responsive | BLCA | Pre-clinical |
| TP53 | N268I | Complete Match | Mitomycin C (Chemotherapy) | Responsive | BLCA | Pre-clinical |
| TP53 | N268I | Complete Match | WEE1 inhibitor | Responsive | HNC | Pre-clinical |
| TP53 | N268I | Complete Match | MDM2 inhibitor | Resistant | LIP | Early trials |
| TP53 | N268I | Complete Match | Doxorubicin (Anthracycline antitumor antibiotic) | Responsive | BLCA | Pre-clinical |
| TP53 | N268I | Complete Match | Pramlintide (Amylin analogue) | Responsive | THYM | Pre-clinical |
| TP53 | N268I | Complete Match | WEE1 inhibitor | Responsive | OV | Early trials |
| TP53 | N268I | Different Alteration | Pramlintide (Amylin analogue) | Responsive | THYM | Pre-clinical |
| TP53 | N268I | Different Alteration | AZD6738 (ATR inhibitor) | Responsive | BCL | Early trials |
| TP53 | Y234* | Different Mutation | HSP90 inhibitor | Responsive | CANCER | Pre-clinical |
| TP53 | Y234* | Complete Match | Decitabine (Chemotherapy) | Responsive | AML, MDPS | Early trials |
| TP53 | Y234* | Complete Match | Abemaciclib (CDK4/6 inhibitor) | Resistant | BRCA | Early trials |
| TP53 | Y234* | Different Mutation | HDM2 inhibitor | Responsive | AML | Early trials |
| TP53 | Y234* | Complete Match | Cisplatin (Chemotherapy) | Resistant | FGCT, MGCT | Early trials |
| TP53 | Y234* | Complete Match | AZD6738 (ATR inhibitor) | Responsive | BCL | Early trials |
| TP53 | Y234* | Complete Match | Gemcitabine (Chemotherapy) | Responsive | BLCA | Pre-clinical |
| TP53 | Y234* | Complete Match | Mitomycin C (Chemotherapy) | Responsive | BLCA | Pre-clinical |
| TP53 | Y234* | Complete Match | WEE1 inhibitor | Responsive | HNC | Pre-clinical |
| TP53 | Y234* | Complete Match | MDM2 inhibitor | Resistant | LIP | Early trials |
| TP53 | Y234* | Complete Match | Doxorubicin (Anthracycline antitumor antibiotic) | Responsive | BLCA | Pre-clinical |
| TP53 | Y234* | Complete Match | Pramlintide (Amylin analogue) | Responsive | THYM | Pre-clinical |
| TP53 | Y234* | Complete Match | WEE1 inhibitor | Responsive | OV | Early trials |
| TP53 | Y234* | Different Alteration | Pramlintide (Amylin analogue) | Responsive | THYM | Pre-clinical |
| TP53 | Y234* | Different Alteration | AZD6738 (ATR inhibitor) | Responsive | BCL | Early trials |
| TP53 | V197M | Different Mutation | HSP90 inhibitor | Responsive | CANCER | Pre-clinical |
| TP53 | V197M | Complete Match | Decitabine (Chemotherapy) | Responsive | AML, MDPS | Early trials |
| TP53 | V197M | Complete Match | Abemaciclib (CDK4/6 inhibitor) | Resistant | BRCA | Early trials |
| TP53 | V197M | Different Mutation | HDM2 inhibitor | Responsive | AML | Early trials |
| TP53 | V197M | Complete Match | Cisplatin (Chemotherapy) | Resistant | FGCT, MGCT | Early trials |
| TP53 | V197M | Complete Match | AZD6738 (ATR inhibitor) | Responsive | BCL | Early trials |
| TP53 | V197M | Complete Match | Gemcitabine (Chemotherapy) | Responsive | BLCA | Pre-clinical |
| TP53 | V197M | Complete Match | Mitomycin C (Chemotherapy) | Responsive | BLCA | Pre-clinical |
| TP53 | V197M | Complete Match | WEE1 inhibitor | Responsive | HNC | Pre-clinical |
| TP53 | V197M | Complete Match | MDM2 inhibitor | Resistant | LIP | Early trials |
| TP53 | V197M | Complete Match | Doxorubicin (Anthracycline antitumor antibiotic) | Responsive | BLCA | Pre-clinical |
| TP53 | V197M | Complete Match | Pramlintide (Amylin analogue) | Responsive | THYM | Pre-clinical |
| TP53 | V197M | Complete Match | WEE1 inhibitor | Responsive | OV | Early trials |
| TP53 | V197M | Different Alteration | Pramlintide (Amylin analogue) | Responsive | THYM | Pre-clinical |
| TP53 | V197M | Different Alteration | AZD6738 (ATR inhibitor) | Responsive | BCL | Early trials |
| TP53 | F134L | Different Mutation | HSP90 inhibitor | Responsive | CANCER | Pre-clinical |
| TP53 | F134L | Complete Match | Decitabine (Chemotherapy) | Responsive | AML, MDPS | Early trials |
| TP53 | F134L | Complete Match | Abemaciclib (CDK4/6 inhibitor) | Resistant | BRCA | Early trials |
| TP53 | F134L | Different Mutation | HDM2 inhibitor | Responsive | AML | Early trials |
| TP53 | F134L | Complete Match | Cisplatin (Chemotherapy) | Resistant | FGCT, MGCT | Early trials |
| TP53 | F134L | Complete Match | AZD6738 (ATR inhibitor) | Responsive | BCL | Early trials |
| TP53 | F134L | Complete Match | Gemcitabine (Chemotherapy) | Responsive | BLCA | Pre-clinical |
| TP53 | F134L | Complete Match | Mitomycin C (Chemotherapy) | Responsive | BLCA | Pre-clinical |
| TP53 | F134L | Complete Match | WEE1 inhibitor | Responsive | HNC | Pre-clinical |
| TP53 | F134L | Complete Match | MDM2 inhibitor | Resistant | LIP | Early trials |
| TP53 | F134L | Complete Match | Doxorubicin (Anthracycline antitumor antibiotic) | Responsive | BLCA | Pre-clinical |
| TP53 | F134L | Complete Match | Pramlintide (Amylin analogue) | Responsive | THYM | Pre-clinical |
| TP53 | F134L | Complete Match | WEE1 inhibitor | Responsive | OV | Early trials |
| TP53 | F134L | Different Alteration | Pramlintide (Amylin analogue) | Responsive | THYM | Pre-clinical |
| TP53 | F134L | Different Alteration | AZD6738 (ATR inhibitor) | Responsive | BCL | Early trials |
| TP53 | Y220*), TP53 MUT (E221* | Different Mutation | HSP90 inhibitor | Responsive | CANCER | Pre-clinical |
| TP53 | Y220*), TP53 MUT (E221* | Complete Match | Decitabine (Chemotherapy) | Responsive | AML, MDPS | Early trials |
| TP53 | Y220*), TP53 MUT (E221* | Complete Match | Abemaciclib (CDK4/6 inhibitor) | Resistant | BRCA | Early trials |
| TP53 | Y220*), TP53 MUT (E221* | Different Mutation | HDM2 inhibitor | Responsive | AML | Early trials |
| TP53 | Y220*), TP53 MUT (E221* | Complete Match | Cisplatin (Chemotherapy) | Resistant | FGCT, MGCT | Early trials |
| TP53 | Y220*), TP53 MUT (E221* | Complete Match | AZD6738 (ATR inhibitor) | Responsive | BCL | Early trials |
| TP53 | Y220*), TP53 MUT (E221* | Complete Match | Gemcitabine (Chemotherapy) | Responsive | BLCA | Pre-clinical |
| TP53 | Y220*), TP53 MUT (E221* | Complete Match | Mitomycin C (Chemotherapy) | Responsive | BLCA | Pre-clinical |
| TP53 | Y220*), TP53 MUT (E221* | Complete Match | WEE1 inhibitor | Responsive | HNC | Pre-clinical |
| TP53 | Y220*), TP53 MUT (E221* | Complete Match | MDM2 inhibitor | Resistant | LIP | Early trials |
| TP53 | Y220*), TP53 MUT (E221* | Complete Match | Doxorubicin (Anthracycline antitumor antibiotic) | Responsive | BLCA | Pre-clinical |
| TP53 | Y220*), TP53 MUT (E221* | Complete Match | Pramlintide (Amylin analogue) | Responsive | THYM | Pre-clinical |
| TP53 | Y220*), TP53 MUT (E221* | Complete Match | WEE1 inhibitor | Responsive | OV | Early trials |
| TP53 | Y220*), TP53 MUT (E221* | Different Alteration | Pramlintide (Amylin analogue) | Responsive | THYM | Pre-clinical |
| TP53 | Y220*), TP53 MUT (E221* | Different Alteration | AZD6738 (ATR inhibitor) | Responsive | BCL | Early trials |
| TP53 | V274A | Different Mutation | HSP90 inhibitor | Responsive | CANCER | Pre-clinical |
| TP53 | V274A | Complete Match | Decitabine (Chemotherapy) | Responsive | AML, MDPS | Early trials |
| TP53 | V274A | Complete Match | Abemaciclib (CDK4/6 inhibitor) | Resistant | BRCA | Early trials |
| TP53 | V274A | Different Mutation | HDM2 inhibitor | Responsive | AML | Early trials |
| TP53 | V274A | Complete Match | Cisplatin (Chemotherapy) | Resistant | FGCT, MGCT | Early trials |
| TP53 | V274A | Complete Match | AZD6738 (ATR inhibitor) | Responsive | BCL | Early trials |
| TP53 | V274A | Complete Match | Gemcitabine (Chemotherapy) | Responsive | BLCA | Pre-clinical |
| TP53 | V274A | Complete Match | Mitomycin C (Chemotherapy) | Responsive | BLCA | Pre-clinical |
| TP53 | V274A | Complete Match | WEE1 inhibitor | Responsive | HNC | Pre-clinical |
| TP53 | V274A | Complete Match | MDM2 inhibitor | Resistant | LIP | Early trials |
| TP53 | V274A | Complete Match | Doxorubicin (Anthracycline antitumor antibiotic) | Responsive | BLCA | Pre-clinical |
| TP53 | V274A | Complete Match | Pramlintide (Amylin analogue) | Responsive | THYM | Pre-clinical |
| TP53 | V274A | Complete Match | WEE1 inhibitor | Responsive | OV | Early trials |
| TP53 | V274A | Different Alteration | Pramlintide (Amylin analogue) | Responsive | THYM | Pre-clinical |
| TP53 | V274A | Different Alteration | AZD6738 (ATR inhibitor) | Responsive | BCL | Early trials |
| TP53 | E346* | Different Mutation | HSP90 inhibitor | Responsive | CANCER | Pre-clinical |
| TP53 | E346* | Complete Match | Decitabine (Chemotherapy) | Responsive | AML, MDPS | Early trials |
| TP53 | E346* | Complete Match | Abemaciclib (CDK4/6 inhibitor) | Resistant | BRCA | Early trials |
| TP53 | E346* | Different Mutation | HDM2 inhibitor | Responsive | AML | Early trials |
| TP53 | E346* | Complete Match | Cisplatin (Chemotherapy) | Resistant | FGCT, MGCT | Early trials |
| TP53 | E346* | Complete Match | AZD6738 (ATR inhibitor) | Responsive | BCL | Early trials |
| TP53 | E346* | Complete Match | Gemcitabine (Chemotherapy) | Responsive | BLCA | Pre-clinical |
| TP53 | E346* | Complete Match | Mitomycin C (Chemotherapy) | Responsive | BLCA | Pre-clinical |
| TP53 | E346* | Complete Match | WEE1 inhibitor | Responsive | HNC | Pre-clinical |
| TP53 | E346* | Complete Match | MDM2 inhibitor | Resistant | LIP | Early trials |
| TP53 | E346* | Complete Match | Doxorubicin (Anthracycline antitumor antibiotic) | Responsive | BLCA | Pre-clinical |
| TP53 | E346* | Complete Match | Pramlintide (Amylin analogue) | Responsive | THYM | Pre-clinical |
| TP53 | E346* | Complete Match | WEE1 inhibitor | Responsive | OV | Early trials |
| TP53 | E346* | Different Alteration | Pramlintide (Amylin analogue) | Responsive | THYM | Pre-clinical |
| TP53 | E346* | Different Alteration | AZD6738 (ATR inhibitor) | Responsive | BCL | Early trials |
| TP53 | C238R | Different Mutation | HSP90 inhibitor | Responsive | CANCER | Pre-clinical |
| TP53 | C238R | Complete Match | Decitabine (Chemotherapy) | Responsive | AML, MDPS | Early trials |
| TP53 | C238R | Complete Match | Abemaciclib (CDK4/6 inhibitor) | Resistant | BRCA | Early trials |
| TP53 | C238R | Different Mutation | HDM2 inhibitor | Responsive | AML | Early trials |
| TP53 | C238R | Complete Match | Cisplatin (Chemotherapy) | Resistant | FGCT, MGCT | Early trials |
| TP53 | C238R | Complete Match | AZD6738 (ATR inhibitor) | Responsive | BCL | Early trials |
| TP53 | C238R | Complete Match | Gemcitabine (Chemotherapy) | Responsive | BLCA | Pre-clinical |
| TP53 | C238R | Complete Match | Mitomycin C (Chemotherapy) | Responsive | BLCA | Pre-clinical |
| TP53 | C238R | Complete Match | WEE1 inhibitor | Responsive | HNC | Pre-clinical |
| TP53 | C238R | Complete Match | MDM2 inhibitor | Resistant | LIP | Early trials |
| TP53 | C238R | Complete Match | Doxorubicin (Anthracycline antitumor antibiotic) | Responsive | BLCA | Pre-clinical |
| TP53 | C238R | Complete Match | Pramlintide (Amylin analogue) | Responsive | THYM | Pre-clinical |
| TP53 | C238R | Complete Match | WEE1 inhibitor | Responsive | OV | Early trials |
| TP53 | C238R | Different Alteration | Pramlintide (Amylin analogue) | Responsive | THYM | Pre-clinical |
| TP53 | C238R | Different Alteration | AZD6738 (ATR inhibitor) | Responsive | BCL | Early trials |
| TP53 | R306* | Different Mutation | HSP90 inhibitor | Responsive | CANCER | Pre-clinical |
| TP53 | R306* | Complete Match | Decitabine (Chemotherapy) | Responsive | AML, MDPS | Early trials |
| TP53 | R306* | Complete Match | Abemaciclib (CDK4/6 inhibitor) | Resistant | BRCA | Early trials |
| TP53 | R306* | Different Mutation | HDM2 inhibitor | Responsive | AML | Early trials |
| TP53 | R306* | Complete Match | Cisplatin (Chemotherapy) | Resistant | FGCT, MGCT | Early trials |
| TP53 | R306* | Complete Match | AZD6738 (ATR inhibitor) | Responsive | BCL | Early trials |
| TP53 | R306* | Complete Match | Gemcitabine (Chemotherapy) | Responsive | BLCA | Pre-clinical |
| TP53 | R306* | Complete Match | Mitomycin C (Chemotherapy) | Responsive | BLCA | Pre-clinical |
| TP53 | R306* | Complete Match | WEE1 inhibitor | Responsive | HNC | Pre-clinical |
| TP53 | R306* | Complete Match | MDM2 inhibitor | Resistant | LIP | Early trials |
| TP53 | R306* | Complete Match | Doxorubicin (Anthracycline antitumor antibiotic) | Responsive | BLCA | Pre-clinical |
| TP53 | R306* | Complete Match | Pramlintide (Amylin analogue) | Responsive | THYM | Pre-clinical |
| TP53 | R306* | Complete Match | WEE1 inhibitor | Responsive | OV | Early trials |
| TP53 | R306* | Different Alteration | Pramlintide (Amylin analogue) | Responsive | THYM | Pre-clinical |
| TP53 | R306* | Different Alteration | AZD6738 (ATR inhibitor) | Responsive | BCL | Early trials |
| TP53 | Q331* | Different Mutation | HSP90 inhibitor | Responsive | CANCER | Pre-clinical |
| TP53 | Q331* | Complete Match | Decitabine (Chemotherapy) | Responsive | AML, MDPS | Early trials |
| TP53 | Q331* | Complete Match | Abemaciclib (CDK4/6 inhibitor) | Resistant | BRCA | Early trials |
| TP53 | Q331* | Different Mutation | HDM2 inhibitor | Responsive | AML | Early trials |
| TP53 | Q331* | Complete Match | Cisplatin (Chemotherapy) | Resistant | FGCT, MGCT | Early trials |
| TP53 | Q331* | Complete Match | AZD6738 (ATR inhibitor) | Responsive | BCL | Early trials |
| TP53 | Q331* | Complete Match | Gemcitabine (Chemotherapy) | Responsive | BLCA | Pre-clinical |
| TP53 | Q331* | Complete Match | Mitomycin C (Chemotherapy) | Responsive | BLCA | Pre-clinical |
| TP53 | Q331* | Complete Match | WEE1 inhibitor | Responsive | HNC | Pre-clinical |
| TP53 | Q331* | Complete Match | MDM2 inhibitor | Resistant | LIP | Early trials |
| TP53 | Q331* | Complete Match | Doxorubicin (Anthracycline antitumor antibiotic) | Responsive | BLCA | Pre-clinical |
| TP53 | Q331* | Complete Match | Pramlintide (Amylin analogue) | Responsive | THYM | Pre-clinical |
| TP53 | Q331* | Complete Match | WEE1 inhibitor | Responsive | OV | Early trials |
| TP53 | Q331* | Different Alteration | Pramlintide (Amylin analogue) | Responsive | THYM | Pre-clinical |
| TP53 | Q331* | Different Alteration | AZD6738 (ATR inhibitor) | Responsive | BCL | Early trials |
| TP53 | P151A | Different Mutation | HSP90 inhibitor | Responsive | CANCER | Pre-clinical |
| TP53 | P151A | Complete Match | Decitabine (Chemotherapy) | Responsive | AML, MDPS | Early trials |
| TP53 | P151A | Complete Match | Abemaciclib (CDK4/6 inhibitor) | Resistant | BRCA | Early trials |
| TP53 | P151A | Different Mutation | HDM2 inhibitor | Responsive | AML | Early trials |
| TP53 | P151A | Complete Match | Cisplatin (Chemotherapy) | Resistant | FGCT, MGCT | Early trials |
| TP53 | P151A | Complete Match | AZD6738 (ATR inhibitor) | Responsive | BCL | Early trials |
| TP53 | P151A | Complete Match | Gemcitabine (Chemotherapy) | Responsive | BLCA | Pre-clinical |
| TP53 | P151A | Complete Match | Mitomycin C (Chemotherapy) | Responsive | BLCA | Pre-clinical |
| TP53 | P151A | Complete Match | WEE1 inhibitor | Responsive | HNC | Pre-clinical |
| TP53 | P151A | Complete Match | MDM2 inhibitor | Resistant | LIP | Early trials |
| TP53 | P151A | Complete Match | Doxorubicin (Anthracycline antitumor antibiotic) | Responsive | BLCA | Pre-clinical |
| TP53 | P151A | Complete Match | Pramlintide (Amylin analogue) | Responsive | THYM | Pre-clinical |
| TP53 | P151A | Complete Match | WEE1 inhibitor | Responsive | OV | Early trials |
| TP53 | P151A | Different Alteration | Pramlintide (Amylin analogue) | Responsive | THYM | Pre-clinical |
| TP53 | P151A | Different Alteration | AZD6738 (ATR inhibitor) | Responsive | BCL | Early trials |
| TP53 | H179Y | Different Mutation | HSP90 inhibitor | Responsive | CANCER | Pre-clinical |
| TP53 | H179Y | Complete Match | Decitabine (Chemotherapy) | Responsive | AML, MDPS | Early trials |
| TP53 | H179Y | Complete Match | Abemaciclib (CDK4/6 inhibitor) | Resistant | BRCA | Early trials |
| TP53 | H179Y | Different Mutation | HDM2 inhibitor | Responsive | AML | Early trials |
| TP53 | H179Y | Complete Match | Cisplatin (Chemotherapy) | Resistant | FGCT, MGCT | Early trials |
| TP53 | H179Y | Complete Match | AZD6738 (ATR inhibitor) | Responsive | BCL | Early trials |
| TP53 | H179Y | Complete Match | Gemcitabine (Chemotherapy) | Responsive | BLCA | Pre-clinical |
| TP53 | H179Y | Complete Match | Mitomycin C (Chemotherapy) | Responsive | BLCA | Pre-clinical |
| TP53 | H179Y | Complete Match | WEE1 inhibitor | Responsive | HNC | Pre-clinical |
| TP53 | H179Y | Complete Match | MDM2 inhibitor | Resistant | LIP | Early trials |
| TP53 | H179Y | Complete Match | Doxorubicin (Anthracycline antitumor antibiotic) | Responsive | BLCA | Pre-clinical |
| TP53 | H179Y | Complete Match | Pramlintide (Amylin analogue) | Responsive | THYM | Pre-clinical |
| TP53 | H179Y | Complete Match | WEE1 inhibitor | Responsive | OV | Early trials |
| TP53 | H179Y | Different Alteration | Pramlintide (Amylin analogue) | Responsive | THYM | Pre-clinical |
| TP53 | H179Y | Different Alteration | AZD6738 (ATR inhibitor) | Responsive | BCL | Early trials |
| TP53 | M246V), TP53 MUT (G245R | Different Mutation | HSP90 inhibitor | Responsive | CANCER | Pre-clinical |
| TP53 | M246V), TP53 MUT (G245R | Complete Match | Decitabine (Chemotherapy) | Responsive | AML, MDPS | Early trials |
| TP53 | M246V), TP53 MUT (G245R | Complete Match | Abemaciclib (CDK4/6 inhibitor) | Resistant | BRCA | Early trials |
| TP53 | M246V), TP53 MUT (G245R | Different Mutation | HDM2 inhibitor | Responsive | AML | Early trials |
| TP53 | M246V), TP53 MUT (G245R | Complete Match | Cisplatin (Chemotherapy) | Resistant | FGCT, MGCT | Early trials |
| TP53 | M246V), TP53 MUT (G245R | Complete Match | AZD6738 (ATR inhibitor) | Responsive | BCL | Early trials |
| TP53 | M246V), TP53 MUT (G245R | Complete Match | Gemcitabine (Chemotherapy) | Responsive | BLCA | Pre-clinical |
| TP53 | M246V), TP53 MUT (G245R | Complete Match | Mitomycin C (Chemotherapy) | Responsive | BLCA | Pre-clinical |
| TP53 | M246V), TP53 MUT (G245R | Complete Match | WEE1 inhibitor | Responsive | HNC | Pre-clinical |
| TP53 | M246V), TP53 MUT (G245R | Complete Match | MDM2 inhibitor | Resistant | LIP | Early trials |
| TP53 | M246V), TP53 MUT (G245R | Complete Match | Doxorubicin (Anthracycline antitumor antibiotic) | Responsive | BLCA | Pre-clinical |
| TP53 | M246V), TP53 MUT (G245R | Complete Match | Pramlintide (Amylin analogue) | Responsive | THYM | Pre-clinical |
| TP53 | M246V), TP53 MUT (G245R | Complete Match | WEE1 inhibitor | Responsive | OV | Early trials |
| TP53 | M246V), TP53 MUT (G245R | Different Alteration | Pramlintide (Amylin analogue) | Responsive | THYM | Pre-clinical |
| TP53 | M246V), TP53 MUT (G245R | Different Alteration | AZD6738 (ATR inhibitor) | Responsive | BCL | Early trials |
| TP53 | Q192H), TP53 MUT (H193Y | Different Mutation | HSP90 inhibitor | Responsive | CANCER | Pre-clinical |
| TP53 | Q192H), TP53 MUT (H193Y | Complete Match | Decitabine (Chemotherapy) | Responsive | AML, MDPS | Early trials |
| TP53 | Q192H), TP53 MUT (H193Y | Complete Match | Abemaciclib (CDK4/6 inhibitor) | Resistant | BRCA | Early trials |
| TP53 | Q192H), TP53 MUT (H193Y | Different Mutation | HDM2 inhibitor | Responsive | AML | Early trials |
| TP53 | Q192H), TP53 MUT (H193Y | Complete Match | Cisplatin (Chemotherapy) | Resistant | FGCT, MGCT | Early trials |
| TP53 | Q192H), TP53 MUT (H193Y | Complete Match | AZD6738 (ATR inhibitor) | Responsive | BCL | Early trials |
| TP53 | Q192H), TP53 MUT (H193Y | Complete Match | Gemcitabine (Chemotherapy) | Responsive | BLCA | Pre-clinical |
| TP53 | Q192H), TP53 MUT (H193Y | Complete Match | Mitomycin C (Chemotherapy) | Responsive | BLCA | Pre-clinical |
| TP53 | Q192H), TP53 MUT (H193Y | Complete Match | WEE1 inhibitor | Responsive | HNC | Pre-clinical |
| TP53 | Q192H), TP53 MUT (H193Y | Complete Match | MDM2 inhibitor | Resistant | LIP | Early trials |
| TP53 | Q192H), TP53 MUT (H193Y | Complete Match | Doxorubicin (Anthracycline antitumor antibiotic) | Responsive | BLCA | Pre-clinical |
| TP53 | Q192H), TP53 MUT (H193Y | Complete Match | Pramlintide (Amylin analogue) | Responsive | THYM | Pre-clinical |
| TP53 | Q192H), TP53 MUT (H193Y | Complete Match | WEE1 inhibitor | Responsive | OV | Early trials |
| TP53 | Q192H), TP53 MUT (H193Y | Different Alteration | Pramlintide (Amylin analogue) | Responsive | THYM | Pre-clinical |
| TP53 | Q192H), TP53 MUT (H193Y | Different Alteration | AZD6738 (ATR inhibitor) | Responsive | BCL | Early trials |
| U2AF1 | S34F | Complete Match | FLT3 inhibitor | Responsive | CANCER | Pre-clinical |
Showing 1 to 3,348 of 3,348 entries
Processing...
| Variation ID | Gene | Transcript | Protein change | Consequence | Driver | Oncogenic classification |
|---|
0
Loading...
Processing...
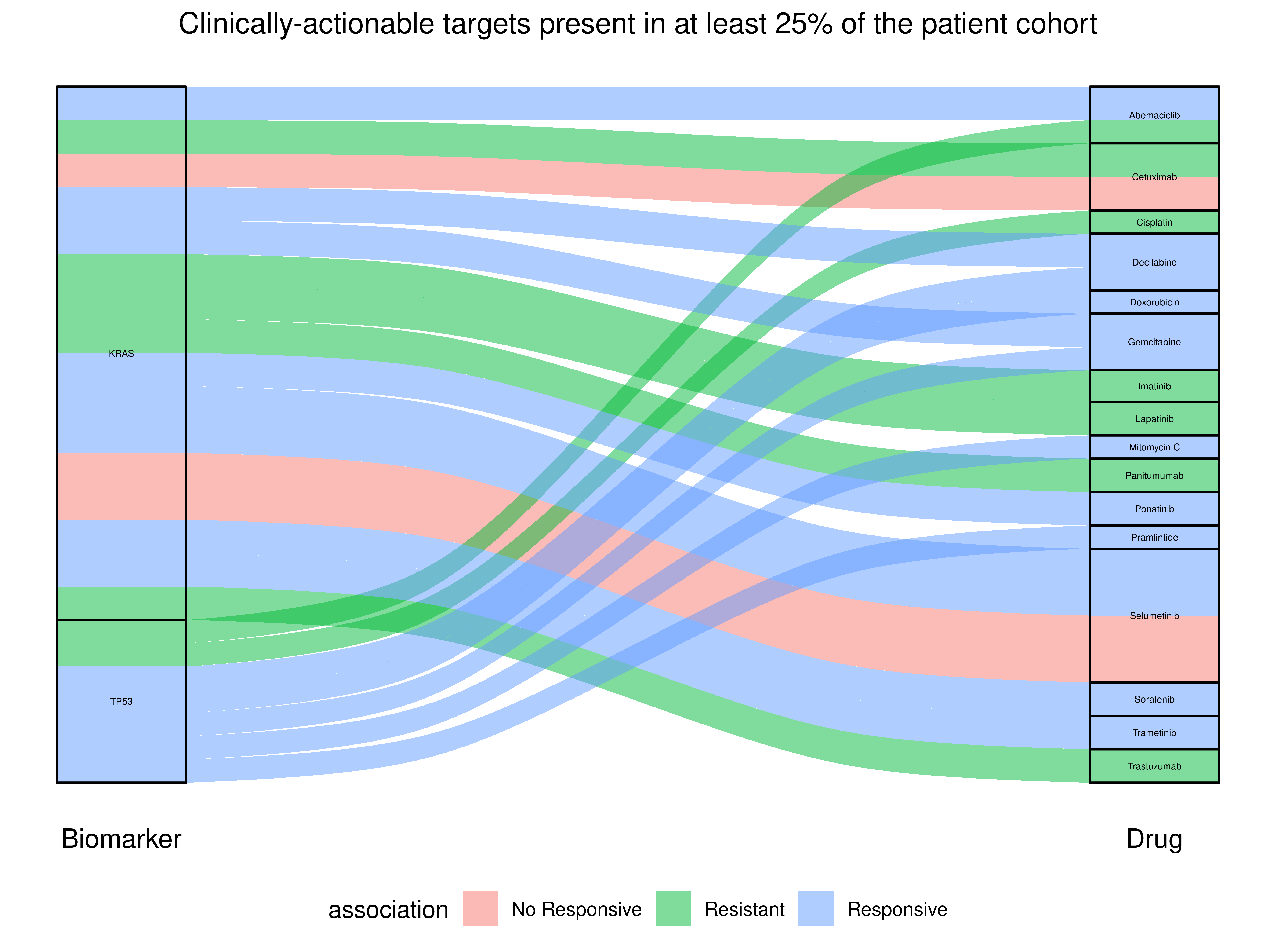
Drug-Gene Interactions help
Druggable categories in the cohort
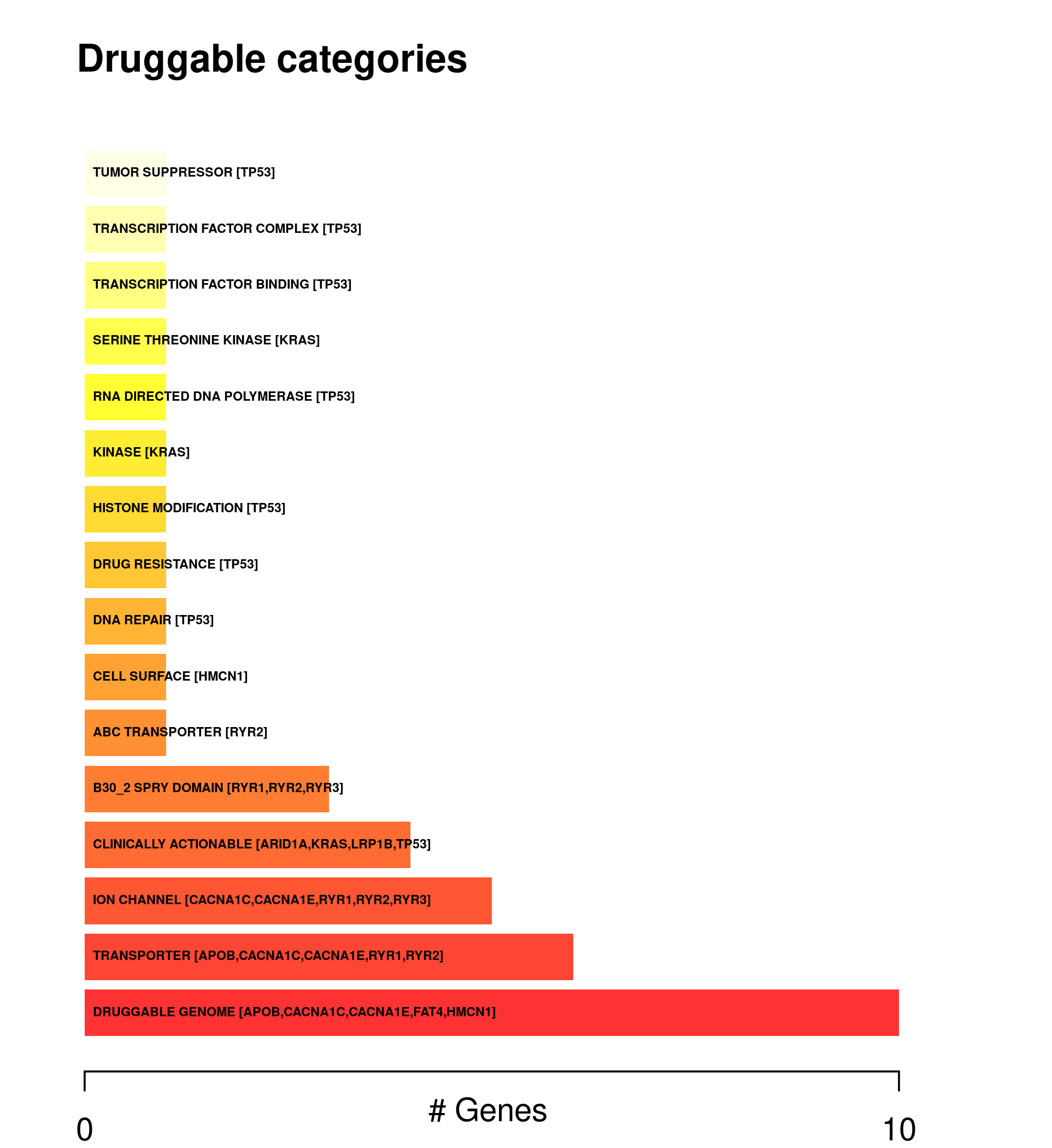
Focus the search to a gene of interest:
Loading...
| Gene | Interaction Source | Interaction Types | Drug Claim Primary Name | Drug Name | ChEMBL ID |
|---|
Gene | Interaction Source | Interaction Types | Drug Claim Primary Name | Drug Name | ChEMBL ID |
|---|---|---|---|---|---|
| KRAS | CKB | lenalidomide | LENALIDOMIDE | CHEMBL848 | |
| KRAS | CKB | GA201 | IMGATUZUMAB | CHEMBL2109389 | |
| KRAS | CKB | 3144 | |||
| KRAS | OncoKB | Selumetinib | SELUMETINIB | CHEMBL1614701 | |
| KRAS | CKB | BKM120 | BUPARLISIB | CHEMBL2017974 | |
| KRAS | CKB | Gefitinib | GEFITINIB | CHEMBL939 | |
| KRAS | CKB | CGM097 | |||
| KRAS | CKB | IRX4204 | IRX-4204 | CHEMBL75133 | |
| KRAS | CKB | MK2206 | MK-2206 | CHEMBL1079175 | |
| KRAS | CKB | Radiotherapy | |||
| KRAS | CGI | Sorafenib | SORAFENIB | CHEMBL1336 | |
| KRAS | CIViC | SCH772984 | |||
| KRAS | CIViC | CISPLATIN | |||
| KRAS | CIViC | ERLOTINIB | ERLOTINIB | CHEMBL553 | |
| KRAS | CIViC | CHEMOTHERAPY | |||
| KRAS | CKB | APS-2-79 | |||
| KRAS | CKB | GDC0879 | CHEMBL525191 | CHEMBL525191 | |
| KRAS | CKB | KO-947 | |||
| KRAS | CIViC | AZD8186 | AZD-8186 | CHEMBL3545424 | |
| KRAS | CKB | Vemurafenib | VEMURAFENIB | CHEMBL1229517 | |
| KRAS | CIViC | PEMBROLIZUMAB | PEMBROLIZUMAB | CHEMBL3137343 | |
| KRAS | CKB | Ipatasertib | IPATASERTIB | CHEMBL2177390 | |
| KRAS | OncoKB | Cabozantinib | CABOZANTINIB | CHEMBL2105717 | |
| KRAS | CKB | Trametinib | TRAMETINIB | CHEMBL2103875 | |
| KRAS | CKB | GI-4000 | |||
| KRAS | ClearityFoundationBiomarkers | PANITUMUMAB | PANITUMUMAB | CHEMBL1201827 | |
| KRAS | CKB | AZD5363 | AZD-5363 | CHEMBL2178577 | |
| KRAS | CKB | LY3009120 | LY-3009120 | CHEMBL3545195 | |
| KRAS | CGI | Lapatinib | LAPATINIB | CHEMBL554 | |
| KRAS | ClearityFoundationBiomarkers | RAFAMETINIB | |||
| KRAS | OncoKB | KO-947 | |||
| KRAS | DoCM | CETUXIMAB | CETUXIMAB | CHEMBL1201577 | |
| KRAS | CKB | GDC-0068 | IPATASERTIB | CHEMBL2177390 | |
| KRAS | CKB | Cetuximab | CETUXIMAB | CHEMBL1201577 | |
| KRAS | CKB | DEL-22379 | |||
| KRAS | CGI | Cetuximab | CETUXIMAB | CHEMBL1201577 | |
| KRAS | CKB | NS1 | |||
| KRAS | CGI | BCL2 inhibitor | |||
| KRAS | CIViC | GDC-0623 | |||
| KRAS | DoCM | PANITUMUMAB | PANITUMUMAB | CHEMBL1201827 | |
| KRAS | CKB | Selumetinib | SELUMETINIB | CHEMBL1614701 | |
| KRAS | CKB | AZD4785 | |||
| KRAS | CKB | PKI-402 | CHEMBL589258 | CHEMBL589258 | |
| KRAS | CIViC | R1507 | TEPROTUMUMAB | CHEMBL1743079 | |
| KRAS | OncoKB | Trametinib | TRAMETINIB | CHEMBL2103875 | |
| KRAS | CIViC | DOCETAXEL | DOCETAXEL | CHEMBL92 | |
| KRAS | CGI | MEK inhibitor | |||
| KRAS | CKB | SAR245409 | VOXTALISIB | CHEMBL3545366 | |
| KRAS | CKB | Romidepsin | ROMIDEPSIN | CHEMBL1213490 | |
| KRAS | CKB | FOLFOX | |||
| KRAS | CKB | AUY922 | NVP-AUY922 | CHEMBL252164 | |
| KRAS | CKB | BGJ398 | INFIGRATINIB | CHEMBL1852688 | |
| KRAS | CKB | ARS-853 | |||
| KRAS | CKB | Panitumumab | PANITUMUMAB | CHEMBL1201827 | |
| KRAS | CIViC | BEZ235 | DACTOLISIB | CHEMBL1879463 | |
| KRAS | CKB | VX-11e | |||
| KRAS | ClearityFoundationBiomarkers | TRAMETINIB | TRAMETINIB | CHEMBL2103875 | |
| KRAS | CKB | UC-857993 | |||
| KRAS | CIViC | BEZ235 (NVP-BEZ235, DACTOLISIB) | DACTOLISIB | CHEMBL1879463 | |
| KRAS | CGI | Regorafenib | REGORAFENIB | CHEMBL1946170 | |
| KRAS | CIViC | GEMCITABINE | GEMCITABINE | CHEMBL888 | |
| KRAS | CKB | Reolysin | |||
| KRAS | CIViC | CETUXIMAB | CETUXIMAB | CHEMBL1201577 | |
| KRAS | CKB | Saracatinib | SARACATINIB | CHEMBL217092 | |
| KRAS | CIViC | PD0325901 | PD-0325901 | CHEMBL507361 | |
| KRAS | CKB | Dinaciclib | DINACICLIB | CHEMBL2103840 | |
| KRAS | OncoKB | Binimetinib | BINIMETINIB | CHEMBL3187723 | |
| KRAS | CKB | AMG 232 | |||
| KRAS | MyCancerGenomeClinicalTrial | inhibitor | REGORAFENIB | REGORAFENIB | CHEMBL1946170 |
| KRAS | CKB | Osimertinib | OSIMERTINIB | CHEMBL3353410 | |
| KRAS | CKB | bortezomib | BORTEZOMIB | CHEMBL325041 | |
| KRAS | OncoKB | LY3214996 | |||
| KRAS | CIViC | ADOPTIVE T-CELL TRANSFER | |||
| KRAS | CKB | Afatinib | AFATINIB | CHEMBL1173655 | |
| KRAS | CKB | Capecitabine | CAPECITABINE | CHEMBL1773 | |
| KRAS | CKB | DHM25 | |||
| KRAS | CKB | PI-3065 | |||
| KRAS | TALC | vaccine | RAS PEPTIDE CANCER VACCINE | ||
| KRAS | OncoKB | Abemaciclib | ABEMACICLIB | CHEMBL3301610 | |
| KRAS | CKB | Sunitinib | SUNITINIB | CHEMBL535 | |
| KRAS | CKB | AT-7867 | CHEMBL428462 | CHEMBL428462 | |
| KRAS | CKB | Abemaciclib | ABEMACICLIB | CHEMBL3301610 | |
| KRAS | CKB | Olaparib | OLAPARIB | CHEMBL521686 | |
| KRAS | CKB | Ponatinib | PONATINIB | CHEMBL1171837 | |
| KRAS | CIViC | AZD5438 | AZD-5438 | CHEMBL488436 | |
| KRAS | CIViC | BEVACIZUMAB | BEVACIZUMAB | CHEMBL1201583 | |
| KRAS | CKB | GDC-0980 | APITOLISIB | CHEMBL1922094 | |
| KRAS | CKB | Alpelisib | ALPELISIB | CHEMBL2396661 | |
| KRAS | CKB | Copanlisib | COPANLISIB | CHEMBL3218576 | |
| KRAS | CKB | Venetoclax | VENETOCLAX | CHEMBL3137309 | |
| KRAS | CKB | Pemetrexed | PEMETREXED (CHEMBL1201258) | CHEMBL1201258 | |
| KRAS | CKB | AZ-TAK1 | |||
| KRAS | CIViC | PACLITAXEL | PACLITAXEL | CHEMBL428647 | |
| KRAS | CKB | MLN2480 | MLN-2480 | CHEMBL3348923 | |
| KRAS | CKB | AZD4547 | AZD-4547 | CHEMBL3184679 | |
| KRAS | CKB | AMG 337 | AMG-337 | CHEMBL3545212 | |
| KRAS | ClearityFoundationBiomarkers | TEMSIROLIMUS | TEMSIROLIMUS | CHEMBL1201182 | |
| KRAS | OncoKB | Atezolizumab | ATEZOLIZUMAB | CHEMBL3707227 | |
| KRAS | CKB | Panobinostat | PANOBINOSTAT | CHEMBL483254 | |
| KRAS | OncoKB | Docetaxel | DOCETAXEL | CHEMBL92 | |
| KRAS | CKB | LY411575 | CHEMBL392068 | CHEMBL392068 | |
| KRAS | CKB | Necitumumab | NECITUMUMAB | CHEMBL1743047 | |
| KRAS | CKB | Phenformin | PHENFORMIN | CHEMBL170988 | |
| KRAS | CGI | Trametinib | TRAMETINIB | CHEMBL2103875 | |
| KRAS | CKB | Oxaliplatin | OXALIPLATIN | CHEMBL414804 | |
| KRAS | CKB | Vistusertib | BENZONATATE | CHEMBL1374379 | |
| KRAS | CKB | LY294002 | LY-294002 | CHEMBL98350 | |
| KRAS | CKB | 7RH | |||
| KRAS | ClearityFoundationBiomarkers | EVEROLIMUS | EVEROLIMUS | CHEMBL1908360 | |
| KRAS | CKB | PD98509 | |||
| KRAS | CKB | Trastuzumab | TRASTUZUMAB | CHEMBL1201585 | |
| KRAS | CKB | AZD9291 | OSIMERTINIB | CHEMBL3353410 | |
| KRAS | CKB | Neratinib | NERATINIB | CHEMBL180022 | |
| KRAS | CKB | Ridaforolimus | RIDAFOROLIMUS | CHEMBL2103839 | |
| KRAS | CKB | A-443654 | A-443654 | CHEMBL379300 | |
| KRAS | CKB | UC-773587 | |||
| KRAS | CKB | Gemcitabine | GEMCITABINE | CHEMBL888 | |
| KRAS | CKB | SAR125844 | SAR-125844 | CHEMBL3545325 | |
| KRAS | CKB | Navitoclax | NAVITOCLAX | CHEMBL443684 | |
| KRAS | CKB | DT-061 | |||
| KRAS | CKB | GSK343 | |||
| KRAS | CKB | GDC-0032 | TASELISIB | CHEMBL2387080 | |
| KRAS | CKB | Binimetinib | BINIMETINIB | CHEMBL3187723 | |
| KRAS | CIViC | GEFITINIB | GEFITINIB | CHEMBL939 | |
| KRAS | CKB | KRAS mutant-specific TIL | |||
| KRAS | CGI | MEK | |||
| KRAS | CKB | DZNep | |||
| KRAS | CKB | AT13148 | AT-13148 | CHEMBL3544960 | |
| KRAS | CKB | Aurora Kinase Inhibitor II | ANILINOQUINAZOLINE1 | CHEMBL382590 | |
| KRAS | CIViC | DECITABINE | DECITABINE | CHEMBL1201129 | |
| KRAS | CKB | Ixabepilone | IXABEPILONE | CHEMBL1201752 | |
| KRAS | CKB | AZD8835 | CANDICIDIN | CHEMBL1200647 | |
| KRAS | CGI | Selumetinib | SELUMETINIB | CHEMBL1614701 | |
| KRAS | CIViC | PEMETREXED | PEMETREXED (CHEMBL1201258) | CHEMBL1201258 | |
| KRAS | CKB | Ralimetinib | RALIMETINIB | CHEMBL2364626 | |
| KRAS | CKB | Pimasertib | PIMASERTIB | CHEMBL2107832 | |
| KRAS | CKB | AG490 | CHEMBL56543 | CHEMBL56543 | |
| KRAS | CKB | Carboplatin | CARBOPLATIN | CHEMBL1351 | |
| KRAS | CKB | CI-1040 | CI-1040 | CHEMBL105442 | |
| KRAS | CKB | PF3644022 | CHEMBL1231206 | CHEMBL1231206 | |
| KRAS | CKB | Paclitaxel | PACLITAXEL | CHEMBL428647 | |
| KRAS | CKB | AZD8186 | AZD-8186 | CHEMBL3545424 | |
| KRAS | CIViC | NIVOLUMAB | NIVOLUMAB | CHEMBL2108738 | |
| KRAS | CKB | Salirasib | SALIRASIB | CHEMBL23293 | |
| KRAS | CKB | Atezolizumab | ATEZOLIZUMAB | CHEMBL3707227 | |
| KRAS | CIViC | ABEMACICLIB | ABEMACICLIB | CHEMBL3301610 | |
| KRAS | CKB | BI2536 | BI-2536 | CHEMBL513909 | |
| KRAS | GuideToPharmacologyInteractions | inhibitor | LONAFARNIB | LONAFARNIB | CHEMBL298734 |
| KRAS | CKB | CC-223 | CC-223 | CHEMBL3545151 | |
| KRAS | CKB | SCH772984 | |||
| KRAS | CKB | Pazopanib | PAZOPANIB | CHEMBL477772 | |
| KRAS | CKB | PF-04691502 | PF-04691502 | CHEMBL1234354 | |
| KRAS | CKB | Cobimetinib | COBIMETINIB | CHEMBL2146883 | |
| KRAS | CKB | CID1067700 | |||
| KRAS | CGI | Panitumumab | PANITUMUMAB | CHEMBL1201827 | |
| KRAS | CIViC | FOLFOX4 | |||
| KRAS | CKB | rigosertib | RIGOSERTIB | CHEMBL1241855 | |
| KRAS | OncoKB | Regorafenib | REGORAFENIB | CHEMBL1946170 | |
| KRAS | CKB | AZD8055 | AZD-8055 | CHEMBL1801204 | |
| KRAS | CGI | Ponatinib | PONATINIB | CHEMBL1171837 | |
| KRAS | CKB | Rilotumumab | RILOTUMUMAB | CHEMBL1743063 | |
| KRAS | OncoKB | Cobimetinib | COBIMETINIB | CHEMBL2146883 | |
| KRAS | CKB | Midostaurin | MIDOSTAURIN | CHEMBL608533 | |
| KRAS | CKB | GDC-0941 | PICTILISIB | CHEMBL521851 | |
| KRAS | CIViC | G-573 | |||
| KRAS | CKB | Lapatinib | LAPATINIB | CHEMBL554 | |
| KRAS | CKB | BEZ235 | DACTOLISIB | CHEMBL1879463 | |
| KRAS | CKB | cabozantinib | CABOZANTINIB | CHEMBL2105717 | |
| KRAS | CKB | Cisplatin | |||
| KRAS | CKB | PHT-427 | |||
| KRAS | CGI | BCL2 | |||
| KRAS | CKB | KPT-185 | |||
| KRAS | CIViC | TRAMETINIB | TRAMETINIB | CHEMBL2103875 | |
| KRAS | OncoKB | GDC-0994 | GDC-0994 | CHEMBL3544964 | |
| KRAS | ClearityFoundationBiomarkers | GEFITINIB | GEFITINIB | CHEMBL939 | |
| KRAS | CKB | OSI-027 | OSI-027 | CHEMBL3120215 | |
| KRAS | OncoKB | Palbociclib | PALBOCICLIB | CHEMBL189963 | |
| KRAS | CKB | cobimetinib | COBIMETINIB | CHEMBL2146883 | |
| KRAS | MyCancerGenomeClinicalTrial | inhibitor | SELUMETINIB | SELUMETINIB | CHEMBL1614701 |
| KRAS | ClearityFoundationBiomarkers | PD-325901 | PD-0325901 | CHEMBL507361 | |
| KRAS | OncoKB | Panitumumab | PANITUMUMAB | CHEMBL1201827 | |
| KRAS | OncoKB | Ribociclib | Ribociclib | CHEMBL3545110 | |
| KRAS | CKB | PF-477736 | PF-00477736 | CHEMBL3545137 | |
| KRAS | CIViC | EGFR INHIBITOR | CHEMBL387187 | CHEMBL387187 | |
| KRAS | CKB | Demcizumab | DEMCIZUMAB | CHEMBL2109384 | |
| KRAS | CKB | Volasertib | VOLASERTIB | CHEMBL1233528 | |
| KRAS | CKB | Pembrolizumab | PEMBROLIZUMAB | CHEMBL3137343 | |
| KRAS | CGI | inhibitor | |||
| KRAS | CKB | SH-1242 | |||
| KRAS | CKB | DS-7423 | DS-7423 | CHEMBL3545248 | |
| KRAS | CKB | BGB-283 | BGB-283 | CHEMBL3545246 | |
| KRAS | CKB | AZ628 | |||
| KRAS | CKB | SHP099 | |||
| KRAS | CKB | GDC-0623 | |||
| KRAS | CKB | ||||
| KRAS | CKB | Sirolimus | SIROLIMUS | CHEMBL413 | |
| KRAS | CKB | Erlotinib | ERLOTINIB | CHEMBL553 | |
| KRAS | CKB | BAY1082439 | BAY-1082439 | CHEMBL3545245 | |
| KRAS | CIViC | ATEZOLIZUMAB | ATEZOLIZUMAB | CHEMBL3707227 | |
| KRAS | CKB | Tazemetostat | |||
| KRAS | CIViC | BINIMETINIB | BINIMETINIB | CHEMBL3187723 | |
| KRAS | CKB | JNJ-42756493 | JNJ-42756493 | CHEMBL3545376 | |
| KRAS | CKB | Regorafenib | REGORAFENIB | CHEMBL1946170 | |
| KRAS | CKB | BYL719 | ALPELISIB | CHEMBL2396661 | |
| KRAS | CKB | Refametinib | Refametinib | CHEMBL2138601 | |
| KRAS | CKB | RO5126766 | |||
| KRAS | CKB | PX-866 | SONOLISIB | CHEMBL411907 | |
| KRAS | CKB | TAE226 | CHEMBL458997 | CHEMBL458997 | |
| KRAS | CKB | Nilotinib | NILOTINIB | CHEMBL255863 | |
| KRAS | CKB | Palbociclib | PALBOCICLIB | CHEMBL189963 | |
| KRAS | CKB | Camptothecin | CAMPTOTHECIN | CHEMBL65 | |
| KRAS | CKB | P7170 | Panulisib | CHEMBL3545322 | |
| KRAS | CKB | Sapanisertib | INK-128 | CHEMBL3545056 | |
| KRAS | CKB | XL147 | PILARALISIB (CHEMBL3218575) | CHEMBL3218575 | |
| KRAS | CKB | Metformin | METFORMIN | CHEMBL1431 | |
| KRAS | CKB | MLN0128 | INK-128 | CHEMBL3545056 | |
| KRAS | OncoKB | Erlotinib | ERLOTINIB | CHEMBL553 | |
| KRAS | CKB | Docetaxel | DOCETAXEL | CHEMBL92 | |
| KRAS | ClearityFoundationBiomarkers | MEK162 | BINIMETINIB | CHEMBL3187723 | |
| KRAS | CIViC | RAF265 | CHIR-265 | CHEMBL558752 | |
| KRAS | CKB | RO4987655 | Ro-4987655 | CHEMBL1614766 | |
| KRAS | CKB | TVB-2640 | |||
| KRAS | CKB | Encorafenib | ENCORAFENIB | CHEMBL3301612 | |
| KRAS | CKB | Danusertib | DANUSERTIB | CHEMBL402548 | |
| KRAS | CKB | MLN1117 | INK-1117 | CHEMBL3545055 | |
| KRAS | CKB | Linsitinib | LINSITINIB | CHEMBL1091644 | |
| KRAS | CIViC | DASATINIB | DASATINIB | CHEMBL1421 | |
| KRAS | CKB | BAY1125976 | BAY-1125976 | CHEMBL3545049 | |
| KRAS | CKB | TW-37 | CHEMBL217354 | CHEMBL217354 | |
| KRAS | CKB | ABT-737 | CHEMBL376408 | CHEMBL376408 | |
| KRAS | MyCancerGenomeClinicalTrial | inhibitor | SIMVASTIN | ||
| KRAS | TdgClinicalTrial | GI-4000 | |||
| KRAS | ClearityFoundationBiomarkers | VANDETANIB | VANDETANIB | CHEMBL24828 | |
| KRAS | CKB | Fluorouracil | FLUOROURACIL | CHEMBL185 | |
| KRAS | CKB | BBI608 | 2-ACETYL FURANONAPTHOQUINONE | CHEMBL64130 | |
| KRAS | CKB | BVD-523 | Ulixertinib | CHEMBL3545022 | |
| KRAS | CKB | Rigosertib | RIGOSERTIB | CHEMBL1241855 | |
| KRAS | CKB | LGX818 | ENCORAFENIB | CHEMBL3301612 | |
| KRAS | ClearityFoundationBiomarkers | GDC-0973 | COBIMETINIB | CHEMBL2146883 | |
| KRAS | CIViC | SELUMETINIB (AZD6244) | SELUMETINIB | CHEMBL1614701 | |
| KRAS | CKB | OPB-111077 | |||
| KRAS | CKB | Dasatinib | DASATINIB | CHEMBL1421 | |
| KRAS | CKB | WEHI-539 | |||
| KRAS | CIViC | PANITUMUMAB | PANITUMUMAB | CHEMBL1201827 | |
| KRAS | CKB | Doxil | |||
| KRAS | CIViC | AFATINIB | AFATINIB | CHEMBL1173655 | |
| KRAS | CKB | BI-847325 | |||
| KRAS | CIViC | CARBOPLATIN | CARBOPLATIN | CHEMBL1351 | |
| KRAS | ClearityFoundationBiomarkers | CETUXIMAB | CETUXIMAB | CHEMBL1201577 | |
| KRAS | CKB | PD-0325901 | PD-0325901 | CHEMBL507361 | |
| KRAS | CGI | Decitabine | DECITABINE | CHEMBL1201129 | |
| KRAS | CKB | BMS-754807 | BMS-754807 | CHEMBL575448 | |
| KRAS | CKB | Deguelin | DEGUELIN | CHEMBL393417 | |
| KRAS | CKB | PF-05212384 | GEDATOLISIB | CHEMBL592445 | |
| KRAS | CKB | fasudil | FASUDIL | CHEMBL38380 | |
| KRAS | CKB | poziotinib | Poziotinib | CHEMBL3545154 | |
| KRAS | CKB | VX-970 | VX-970 | CHEMBL3545202 | |
| KRAS | CGI | Imatinib | IMATINIB | CHEMBL941 | |
| KRAS | CKB | Everolimus | EVEROLIMUS | CHEMBL1908360 | |
| KRAS | CancerCommons | inhibitor | REOLYSIN | ||
| KRAS | CKB | CUDC-907 | CUDC-907 | CHEMBL3545052 | |
| KRAS | ClearityFoundationBiomarkers | ERLOTINIB | ERLOTINIB | CHEMBL553 | |
| KRAS | CKB | MK-1775 | AZD-1775 | CHEMBL1976040 | |
| KRAS | CKB | Aphanin | |||
| KRAS | ClearityFoundationBiomarkers | PIMASERTIB | PIMASERTIB | CHEMBL2107832 | |
| KRAS | ClearityFoundationBiomarkers | SELUMETINIB | SELUMETINIB | CHEMBL1614701 | |
| KRAS | CKB | Irinotecan | IRINOTECAN | CHEMBL481 | |
| KRAS | CGI | Trastuzumab | TRASTUZUMAB | CHEMBL1201585 | |
| KRAS | CIViC | ARRY-142886 | SELUMETINIB | CHEMBL1614701 | |
| KRAS | CKB | Ramucirumab | RAMUCIRUMAB | CHEMBL1743062 | |
| KRAS | CKB | SML-10-70-1 | |||
| KRAS | CKB | MEK162 | BINIMETINIB | CHEMBL3187723 | |
| KRAS | CKB | FOLFIRI | CETUXIMAB | CHEMBL1201577 | |
| KRAS | CIViC | ARS-853 | |||
| KRAS | CKB | ER2 | |||
| KRAS | CKB | Temsirolimus | TEMSIROLIMUS | CHEMBL1201182 | |
| KRAS | CKB | Sorafenib | SORAFENIB | CHEMBL1336 | |
| KRAS | CGI | Docetaxel | DOCETAXEL | CHEMBL92 | |
| KRAS | OncoKB | Cetuximab | CETUXIMAB | CHEMBL1201577 | |
| KRAS | CGI | Palbociclib | PALBOCICLIB | CHEMBL189963 | |
| KRAS | CIViC | ENCORAFENIB | ENCORAFENIB | CHEMBL3301612 | |
| KRAS | CKB | Crizotinib | CRIZOTINIB | CHEMBL601719 | |
| KRAS | CKB | Dabrafenib | DABRAFENIB | CHEMBL2028663 | |
| KRAS | CKB | GSK1120212 | TRAMETINIB | CHEMBL2103875 | |
| KRAS | CIViC | PALBOCICLIB | PALBOCICLIB | CHEMBL189963 | |
| KRAS | CGI | Abemaciclib | ABEMACICLIB | CHEMBL3301610 | |
| KRAS | CKB | SRA737 | |||
| KRAS | CKB | Bevacizumab | BEVACIZUMAB | CHEMBL1201583 | |
| KRAS | CKB | CC-90003 | |||
| KRAS | CKB | TAK-733 | TAK-733 | CHEMBL1615025 | |
| KRAS | CKB | Gedatolisib | GEDATOLISIB | CHEMBL592445 | |
| KRAS | CKB | XMT-1536 | |||
| KRAS | CGI | Gemcitabine | GEMCITABINE | CHEMBL888 | |
| KRAS | CKB | ABT-263 | NAVITOCLAX | CHEMBL443684 | |
| KRAS | CKB | CH5132799 | PA-799 | CHEMBL1684984 |
Showing 1 to 295 of 295 entries
Processing...
Principal Component Analysis
PCA Help
Scatterplot of Two main Principal Components
Click on legend to highlight trace
Scatterplot of Three main Principal Components
Gene Expression
Correlation
Survival Analysis
There are Blood (Plasma, Serum, Buffy coat), FFPE tissue, Fresh frozen tissue samples from unique donors available at PCRFTB, based on the selected clinical filters (Age, Sex, Race, Diagnosis). Researchers can request for samples by submitting an Expression of Interest to PCRFTB.
Overview
Sex
Race
Diagnosis
Tumor stage
Age at Diagnosis



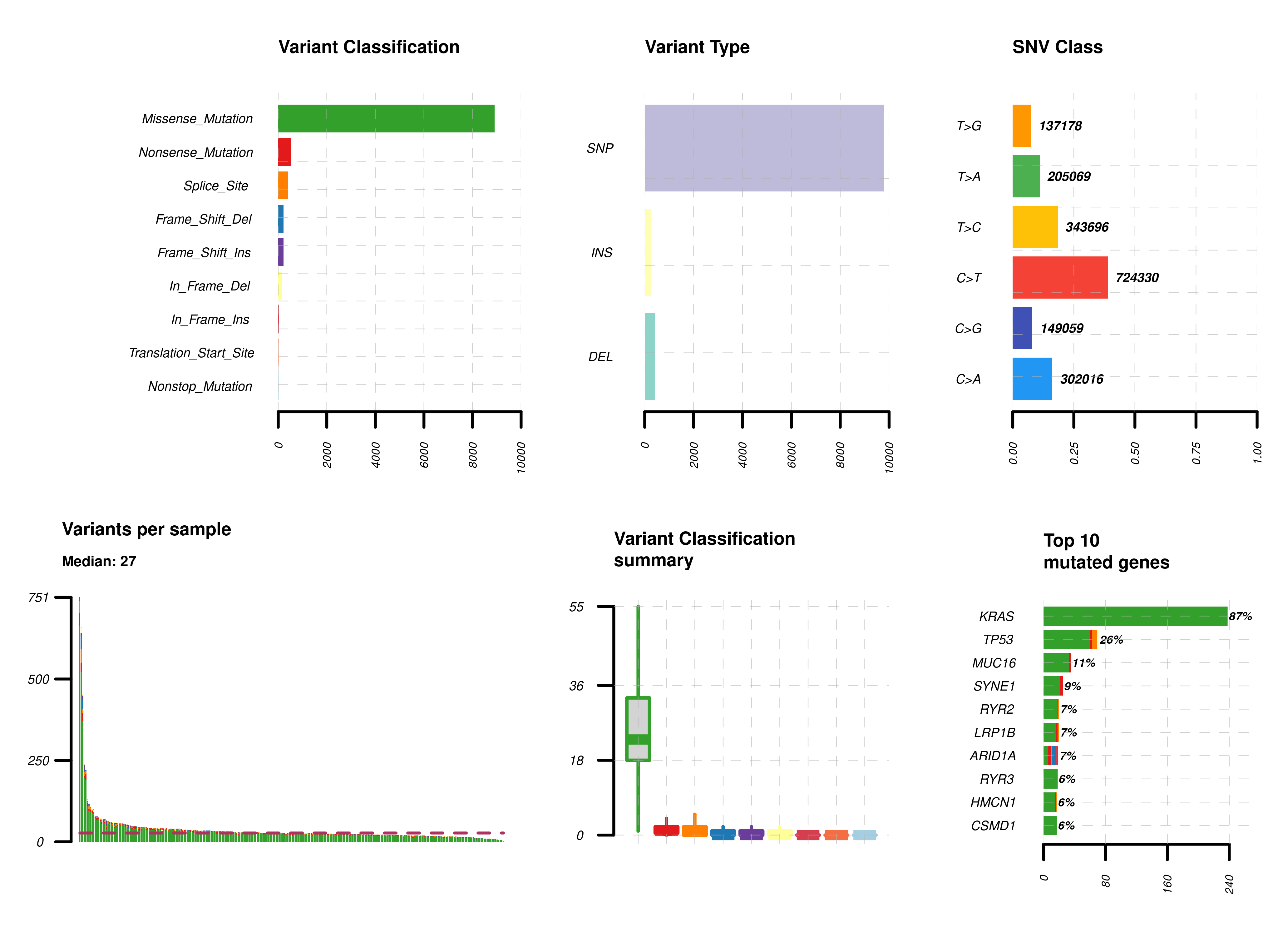
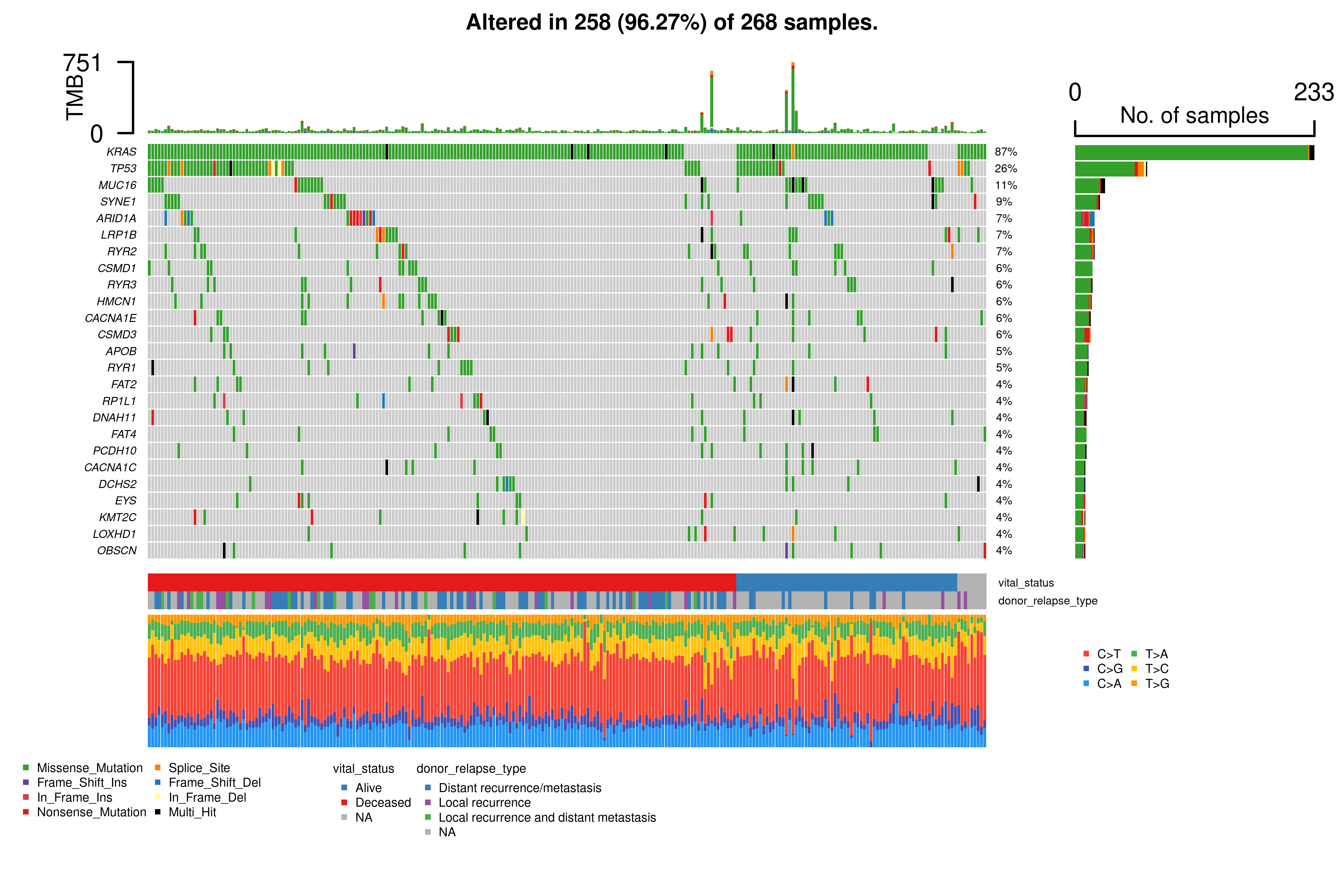
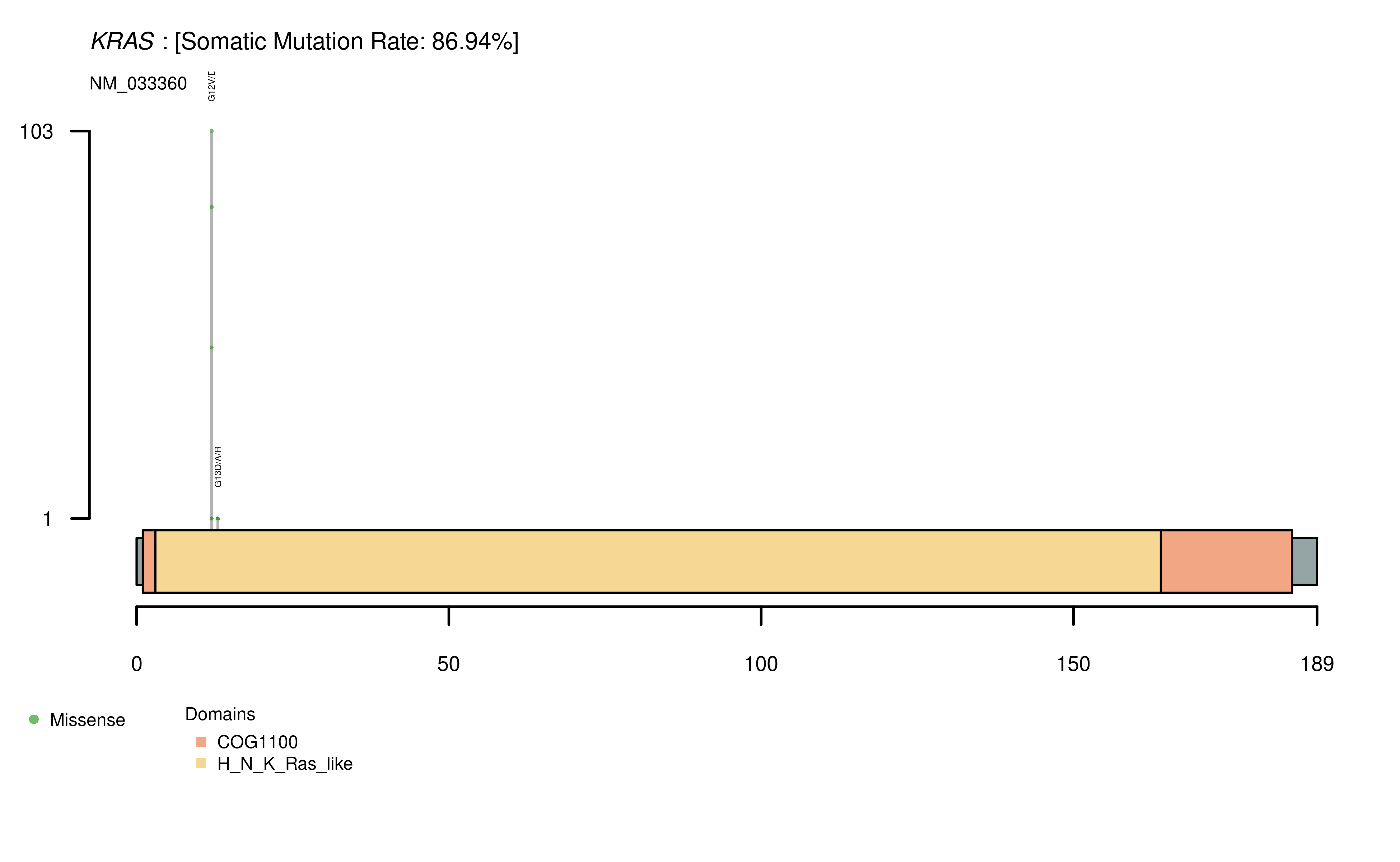
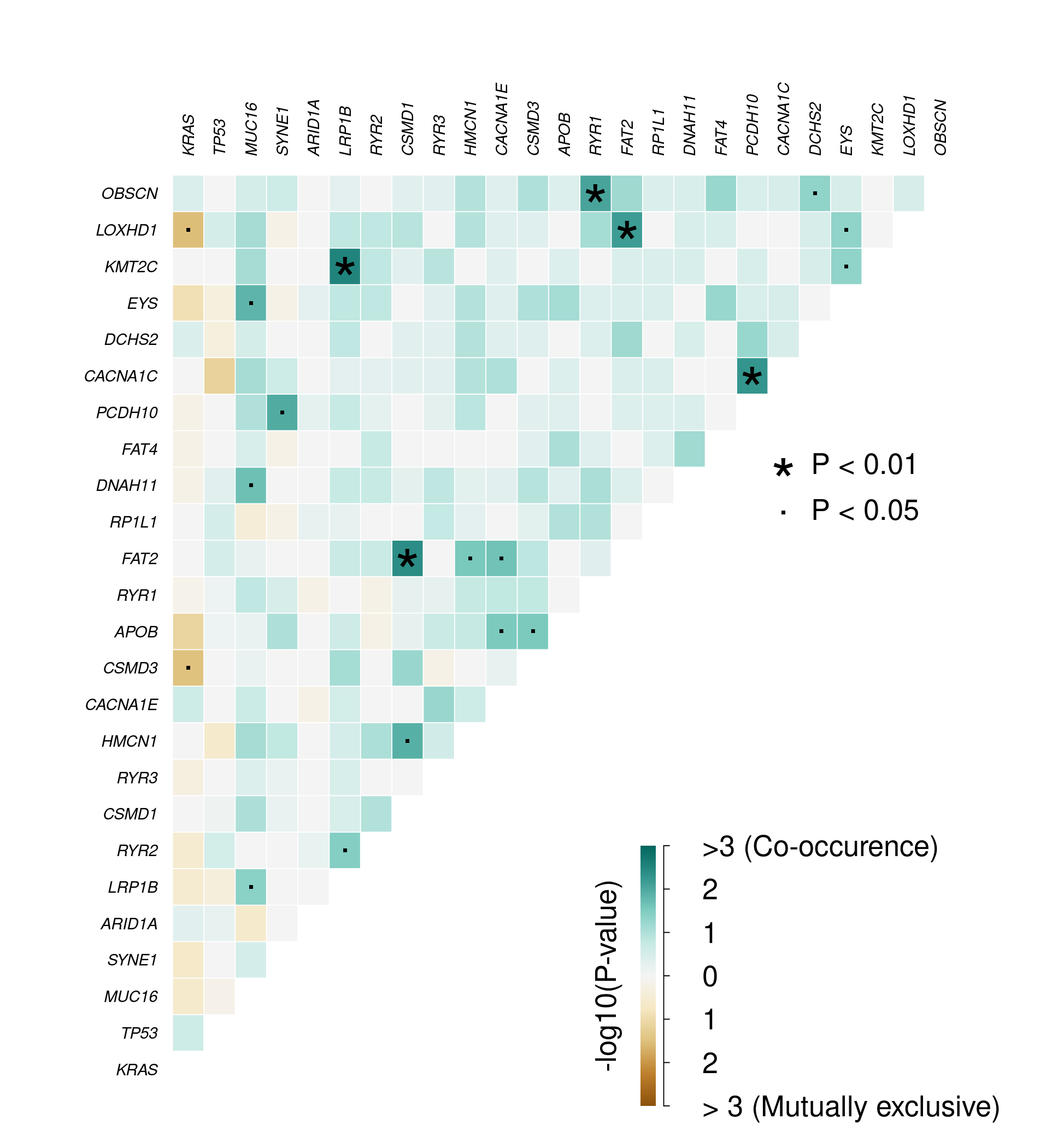
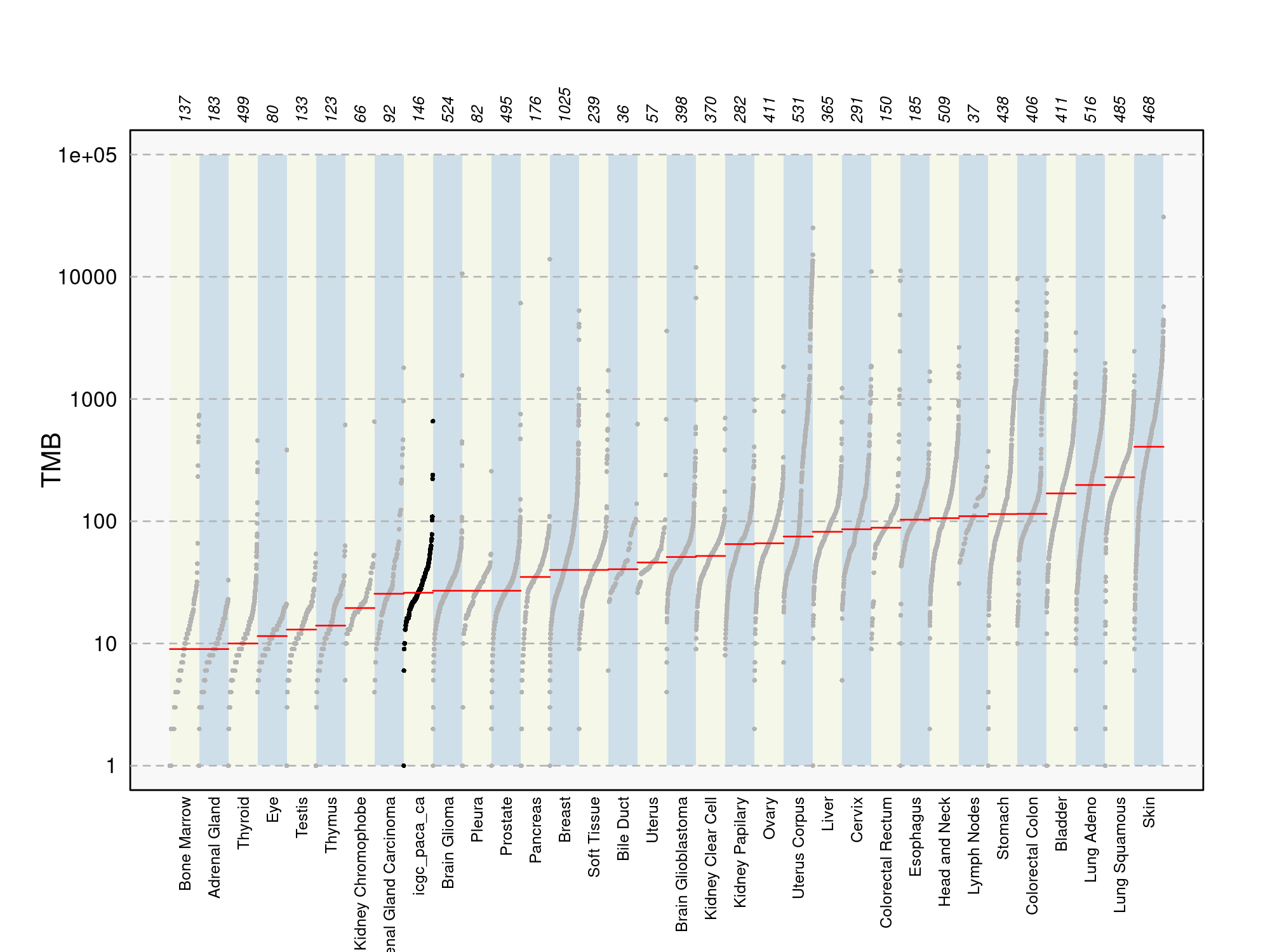
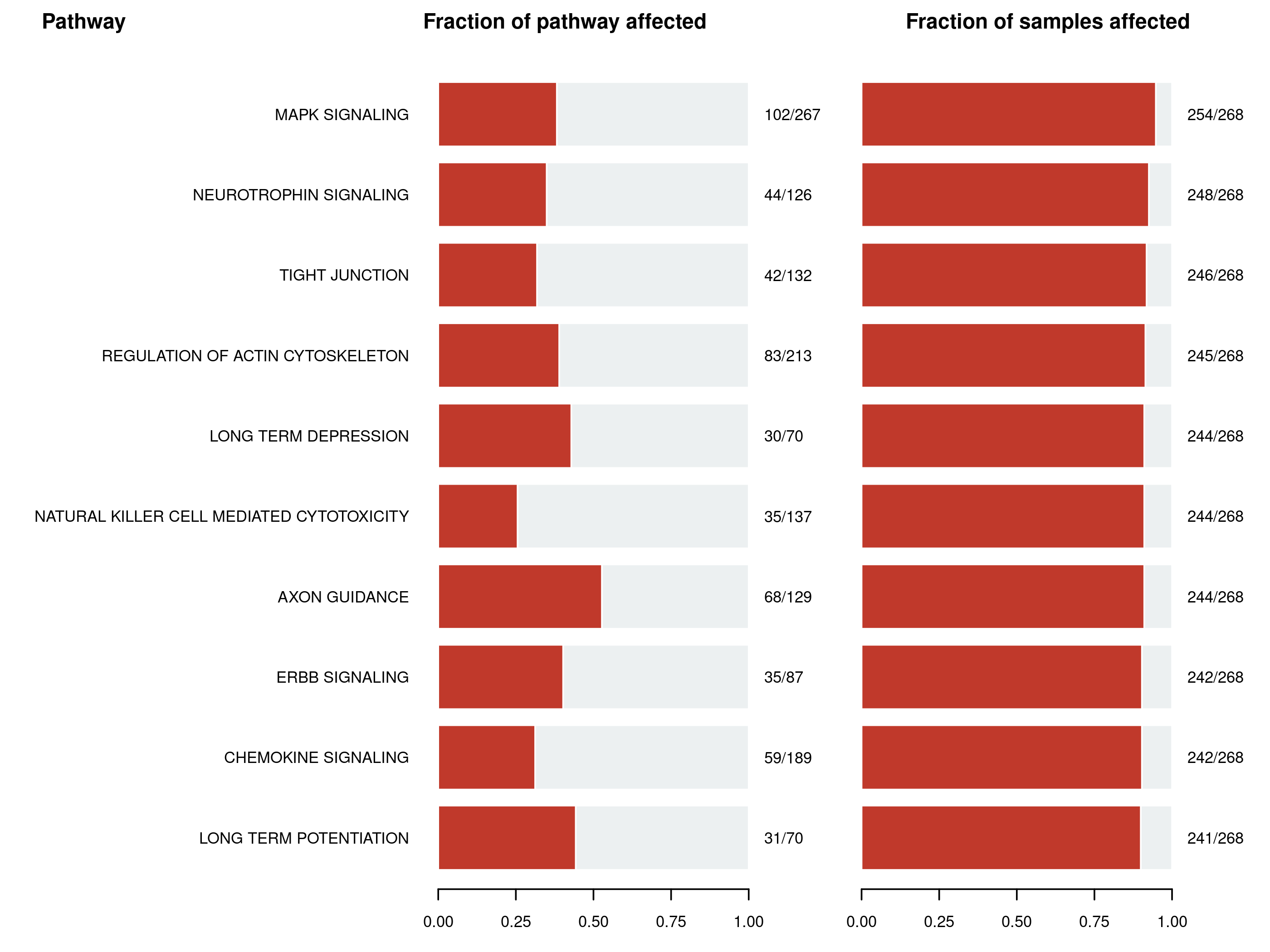
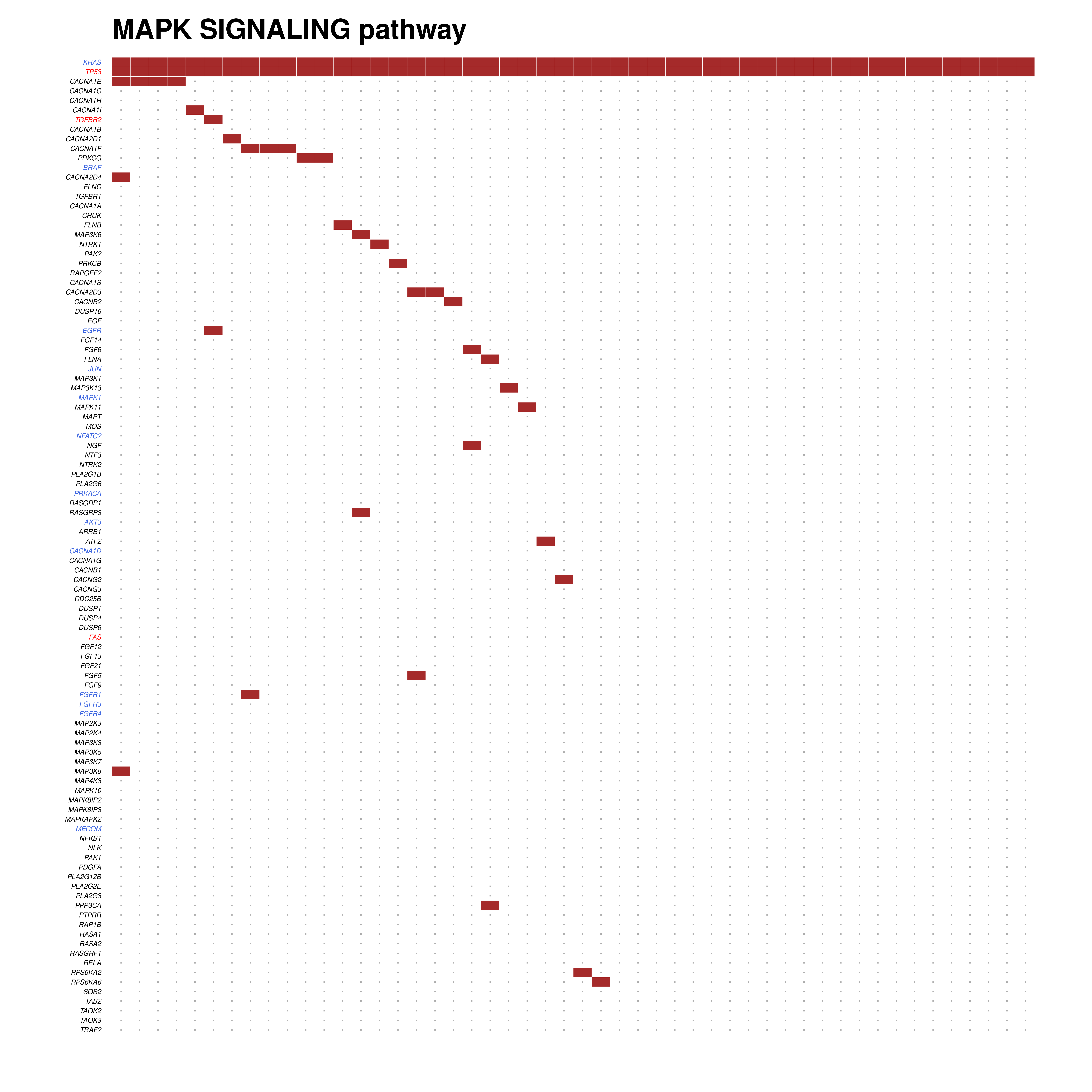

 Phenotype
Phenotype Protein
Protein Drug
Drug