| ERBB4 |
intron_variant), ERBB4 MUT* (intron_variant |
Different Alteration |
Lapatinib;Afatinib (ERBB2 inhibitor;ERBB2 & EGFR inhibitor) |
Responsive |
LUAD |
Pre-clinical |
| ALK |
intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant |
Different Alteration |
ALK inhibitor;SRC inhibitor |
Responsive |
LUAD |
Pre-clinical |
| ALK |
intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant |
Different Alteration |
Crizotinib (ALK inhibitor) |
Responsive |
GB, LY |
Early trials |
| ALK |
intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant |
Different Alteration |
Lorlatinib (ALK&ROS1 inhibitor) |
Responsive |
NSCLC |
Early trials |
| ALK |
intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant |
Different Alteration |
Crizotinib (ALK inhibitor) |
Responsive |
THCA, IM |
Case report |
| ALK |
intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant |
Different Alteration |
HSP90 inhibitor |
Responsive |
LUAD |
Early trials |
| ALK |
intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant |
Different Alteration |
Ceritinib (ALK inhibitor) |
Responsive |
LUAD, NSCLC |
FDA guidelines |
| ALK |
intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant |
Different Alteration |
Ceritinib (ALK inhibitor) |
Responsive |
IM, COREAD |
Case report |
| ALK |
intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant |
Different Alteration |
Crizotinib (ALK inhibitor) |
Responsive |
LUAD, NSCLC |
FDA guidelines |
| ALK |
intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant |
Different Alteration |
Brigatinib (Pan-TK inhibitor) |
Responsive |
NSCLC |
FDA guidelines |
| ALK |
intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant |
Different Alteration |
Entrictinib (Pan-TK inhibitor) |
Responsive |
COREAD |
Case report |
| ALK |
intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant |
Different Alteration |
Alectinib (ALK inhibitor) |
Responsive |
LUAD |
FDA guidelines |
| ALK |
intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant |
Different Alteration |
HSP90 inhibitor (HSP90 inhibitor) |
Responsive |
LUAD |
Early trials |
| ALK |
intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant |
Different Alteration |
ALK inhibitor;IGF1R inhibitor |
Responsive |
LUAD |
Pre-clinical |
| ALK |
intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant |
Different Alteration |
ALK inhibitor;MEK inhibitor |
Responsive |
LUAD |
Pre-clinical |
| ALK |
intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant |
Different Alteration |
novel ALK inhibitor |
Responsive |
LUAD |
Early trials |
| ALK |
intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant |
Different Alteration |
Ceritinib (ALK inhibitor) |
Responsive |
HEMATO |
Early trials |
| ALK |
intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant |
Different Alteration |
ALK inhibitor |
Responsive |
COREAD |
Case report |
| ALK |
intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant |
Different Alteration |
Alectinib (ALK inhibitor) |
Responsive |
NSCLC |
FDA guidelines |
| ALK |
intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant |
Different Alteration |
MEK inhibitor (ALK inhibitor;MEK inhibitor) |
Responsive |
LUAD |
Pre-clinical |
| ARID1A |
Q1584* |
Complete Match |
EZH2 inhibitor |
Responsive |
OV |
Pre-clinical |
| ARID1A |
Q1584* |
Complete Match |
PD1 inhibitor |
Responsive |
OV |
Pre-clinical |
| ARID1A |
Q1584* |
Complete Match |
PARP inhibitor |
Responsive |
CANCER |
Pre-clinical |
| ARID1A |
Q1584* |
Complete Match |
ATR inhibitor |
Responsive |
CANCER |
Pre-clinical |
| PTEN |
R233* |
Different Mutation |
Cetuximab (EGFR mAb inhibitor) |
Resistant |
COREAD |
Case report |
| PTEN |
R233* |
Different Mutation |
Panitumumab (EGFR mAb inhibitor) |
Resistant |
COREAD |
Case report |
| PTEN |
R233* |
Different Mutation |
BYL719 (PIK3CA inhibitor) |
Resistant |
BRCA |
Case report |
| PTEN |
R233* |
Complete Match |
ATM inhibitor |
Responsive |
BRCA |
Pre-clinical |
| PTEN |
R233* |
Complete Match |
PI3K pathway inhibitor |
Responsive |
BRCA, ED, TH, CANCER, L, G, OV |
Pre-clinical |
| PTEN |
R233* |
Complete Match |
EGFR mAb inhibitor |
Resistant |
COREAD |
Late trials |
| PTEN |
R233* |
Complete Match |
Sirolimus (MTOR inhibitor) |
Responsive |
CANCER |
Early trials |
| PTEN |
R233* |
Complete Match |
PARP inhibitor |
Responsive |
ED |
Case report |
| PTEN |
R233* |
Complete Match |
PD1 Ab inhibitor |
Resistant |
CM |
Early trials |
| PTEN |
R233* |
Complete Match |
PI3K pathway inhibitor;AR antagonist |
Responsive |
PRAD |
Pre-clinical |
| PTEN |
R233* |
Complete Match |
Everolimus (MTOR inhibitor) |
Responsive |
PRAD |
Early trials |
| PTEN |
R233* |
Complete Match |
PI3K pathway inhibitor;MEK inhibitor |
Responsive |
OV |
Pre-clinical |
| PTEN |
R233* |
Complete Match |
PIK3CB inhibitor |
Responsive |
PRAD |
Case report |
| PTEN |
R233* |
Complete Match |
AKT inhibitor |
Responsive |
PA |
Case report |
| PTEN |
R233* |
Complete Match |
MTOR inhibitor |
No Responsive |
ED |
Early trials |
| PTEN |
R233* |
Different Alteration |
PI3K pathway inhibitor |
Responsive |
L, BRCA, CANCER, G, ED, TH, OV |
Pre-clinical |
| PTEN |
R233* |
Different Alteration |
Everolimus (MTOR inhibitor) |
Responsive |
PRAD |
Early trials |
| PTEN |
R233* |
Different Alteration |
Sirolimus (MTOR inhibitor) |
Responsive |
CANCER |
Early trials |
| PTEN |
R233* |
Different Alteration |
PARP inhibitor |
Responsive |
ED |
Case report |
| PTEN |
R233* |
Different Alteration |
PIK3CB inhibitor |
Responsive |
PRAD |
Case report |
| PTEN |
R233* |
Different Alteration |
EGFR mAb inhibitor |
Resistant |
COREAD |
Late trials |
| PTEN |
R233* |
Different Alteration |
MTOR inhibitor |
No Responsive |
ED |
Early trials |
| PTEN |
R233* |
Different Alteration |
AKT inhibitor |
Responsive |
PA |
Case report |
| PTEN |
R233* |
Different Alteration |
PI3K pathway inhibitor;AR antagonist |
Responsive |
PRAD |
Pre-clinical |
| PTEN |
R233* |
Different Alteration |
PI3K pathway inhibitor;MEK inhibitor |
Responsive |
OV |
Pre-clinical |
| PDGFRA |
intron_variant), PDGFRA MUT* (intron_variant |
Different Alteration |
Imatinib (BCR-ABL inhibitor & KIT inhibitor) |
Responsive |
MDPS, MDS, ECL, HES |
FDA guidelines |
| BRAF |
intron_variant |
Different Alteration |
Sorafenib (Pan-TK inhibitor) |
Responsive |
CM, LUAD, PRAD |
Pre-clinical |
| BRAF |
intron_variant |
Different Alteration |
MEK inhibitor |
Responsive |
LUAD, CM, PRAD |
Pre-clinical |
| BRAF |
intron_variant |
Different Alteration |
Selumetinib (MEK inhibitor) |
Responsive |
OV |
Case report |
| BRAF |
intron_variant |
Different Alteration |
BRAF inhibitor;MEK inhibitor |
Responsive |
LUAD |
Case report |
| ESR1 |
intron_variant |
Different Alteration |
ESR1 inhibitor |
Resistant |
BRCA |
Pre-clinical |
| ERBB4 |
intron_variant |
Different Alteration |
Lapatinib;Afatinib (ERBB2 inhibitor;ERBB2 & EGFR inhibitor) |
Responsive |
LUAD |
Pre-clinical |
| NRG1 |
intron_variant |
Different Alteration |
Lapatinib (ERBB2 inhibitor) |
Responsive |
LUAD |
Pre-clinical |
| NRG1 |
intron_variant |
Different Alteration |
Afatinib (ERBB2 & EGFR inhibitor) |
Responsive |
LUAD |
Case report |
| ESR1 |
intron_variant), ESR1 MUT* (intron_variant), ESR1 MUT* (intron_variant), ESR1 MUT* (intron_variant |
Different Alteration |
ESR1 inhibitor |
Resistant |
BRCA |
Pre-clinical |
| ERBB4 |
intron_variant), ERBB4 MUT* (intron_variant), ERBB4 MUT* (intron_variant), ERBB4 MUT* (intron_variant), ERBB4 MUT* (intron_variant), ERBB4 MUT* (intron_variant), ERBB4 MUT* (intron_variant), ERBB4 MUT* (intron_variant), ERBB4 MUT* (intron_variant), E |
Different Alteration |
Lapatinib;Afatinib (ERBB2 inhibitor;ERBB2 & EGFR inhibitor) |
Responsive |
LUAD |
Pre-clinical |
| EGFR |
intron_variant |
Different Alteration |
Erlotinib (EGFR inhibitor) |
Responsive |
L |
Case report |
| EGFR |
intron_variant |
Different Alteration |
Erlotinib (EGFR inhibitor) |
Responsive |
NSCLC |
Case report |
| ALK |
intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant |
Different Alteration |
ALK inhibitor;SRC inhibitor |
Responsive |
LUAD |
Pre-clinical |
| ALK |
intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant |
Different Alteration |
Crizotinib (ALK inhibitor) |
Responsive |
GB, LY |
Early trials |
| ALK |
intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant |
Different Alteration |
Lorlatinib (ALK&ROS1 inhibitor) |
Responsive |
NSCLC |
Early trials |
| ALK |
intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant |
Different Alteration |
Crizotinib (ALK inhibitor) |
Responsive |
THCA, IM |
Case report |
| ALK |
intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant |
Different Alteration |
HSP90 inhibitor |
Responsive |
LUAD |
Early trials |
| ALK |
intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant |
Different Alteration |
Ceritinib (ALK inhibitor) |
Responsive |
LUAD, NSCLC |
FDA guidelines |
| ALK |
intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant |
Different Alteration |
Ceritinib (ALK inhibitor) |
Responsive |
IM, COREAD |
Case report |
| ALK |
intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant |
Different Alteration |
Crizotinib (ALK inhibitor) |
Responsive |
LUAD, NSCLC |
FDA guidelines |
| ALK |
intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant |
Different Alteration |
Brigatinib (Pan-TK inhibitor) |
Responsive |
NSCLC |
FDA guidelines |
| ALK |
intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant |
Different Alteration |
Entrictinib (Pan-TK inhibitor) |
Responsive |
COREAD |
Case report |
| ALK |
intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant |
Different Alteration |
Alectinib (ALK inhibitor) |
Responsive |
LUAD |
FDA guidelines |
| ALK |
intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant |
Different Alteration |
HSP90 inhibitor (HSP90 inhibitor) |
Responsive |
LUAD |
Early trials |
| ALK |
intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant |
Different Alteration |
ALK inhibitor;IGF1R inhibitor |
Responsive |
LUAD |
Pre-clinical |
| ALK |
intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant |
Different Alteration |
ALK inhibitor;MEK inhibitor |
Responsive |
LUAD |
Pre-clinical |
| ALK |
intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant |
Different Alteration |
novel ALK inhibitor |
Responsive |
LUAD |
Early trials |
| ALK |
intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant |
Different Alteration |
Ceritinib (ALK inhibitor) |
Responsive |
HEMATO |
Early trials |
| ALK |
intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant |
Different Alteration |
ALK inhibitor |
Responsive |
COREAD |
Case report |
| ALK |
intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant |
Different Alteration |
Alectinib (ALK inhibitor) |
Responsive |
NSCLC |
FDA guidelines |
| ALK |
intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant |
Different Alteration |
MEK inhibitor (ALK inhibitor;MEK inhibitor) |
Responsive |
LUAD |
Pre-clinical |
| ABL1 |
intron_variant), ABL1 MUT* (intron_variant |
Different Alteration |
Ponatinib (BCR-ABL inhibitor & Pan-TK inhibitor) |
Responsive |
CML, ALL |
FDA guidelines |
| ABL1 |
intron_variant), ABL1 MUT* (intron_variant |
Different Alteration |
Dasatinib (BCR-ABL inhibitor) |
Responsive |
ALL, CML |
FDA guidelines |
| ABL1 |
intron_variant), ABL1 MUT* (intron_variant |
Different Alteration |
Nilotinib (BCR-ABL inhibitor) |
Responsive |
CML |
FDA guidelines |
| ABL1 |
intron_variant), ABL1 MUT* (intron_variant |
Different Alteration |
Imatinib (BCR-ABL inhibitor & KIT inhibitor) |
Responsive |
ALL, CML |
FDA guidelines |
| ABL1 |
intron_variant), ABL1 MUT* (intron_variant |
Different Alteration |
Dasatinib;Venetoclax (BCR-ABL inhibitor;BCL2 inhibitor) |
Responsive |
ALL |
Pre-clinical |
| ABL1 |
intron_variant), ABL1 MUT* (intron_variant |
Different Alteration |
Bosutinib (BCR-ABL inhibitor) |
Responsive |
CML |
FDA guidelines |
| FGFR2 |
intron_variant |
Different Alteration |
FGFR inhibitor |
Responsive |
COREAD |
Early trials |
| FGFR2 |
intron_variant |
Different Alteration |
FGFR inhibitor |
Responsive |
BT |
Early trials |
| TP53 |
R248Q |
Complete Match |
HSP90 inhibitor |
Responsive |
CANCER |
Pre-clinical |
| TP53 |
R248Q |
Complete Match |
Decitabine (Chemotherapy) |
Responsive |
AML, MDPS |
Early trials |
| TP53 |
R248Q |
Complete Match |
Abemaciclib (CDK4/6 inhibitor) |
Resistant |
BRCA |
Early trials |
| TP53 |
R248Q |
Different Mutation |
HDM2 inhibitor |
Responsive |
AML |
Early trials |
| TP53 |
R248Q |
Complete Match |
Cisplatin (Chemotherapy) |
Resistant |
FGCT, MGCT |
Early trials |
| TP53 |
R248Q |
Complete Match |
AZD6738 (ATR inhibitor) |
Responsive |
BCL |
Early trials |
| TP53 |
R248Q |
Complete Match |
Gemcitabine (Chemotherapy) |
Responsive |
BLCA |
Pre-clinical |
| TP53 |
R248Q |
Complete Match |
Mitomycin C (Chemotherapy) |
Responsive |
BLCA |
Pre-clinical |
| TP53 |
R248Q |
Complete Match |
WEE1 inhibitor |
Responsive |
HNC |
Pre-clinical |
| TP53 |
R248Q |
Complete Match |
MDM2 inhibitor |
Resistant |
LIP |
Early trials |
| TP53 |
R248Q |
Complete Match |
Doxorubicin (Anthracycline antitumor antibiotic) |
Responsive |
BLCA |
Pre-clinical |
| TP53 |
R248Q |
Complete Match |
Pramlintide (Amylin analogue) |
Responsive |
THYM |
Pre-clinical |
| TP53 |
R248Q |
Complete Match |
WEE1 inhibitor |
Responsive |
OV |
Early trials |
| TP53 |
R248Q |
Different Alteration |
Pramlintide (Amylin analogue) |
Responsive |
THYM |
Pre-clinical |
| TP53 |
R248Q |
Different Alteration |
AZD6738 (ATR inhibitor) |
Responsive |
BCL |
Early trials |
| ROS1 |
intron_variant), ROS1 MUT* (intron_variant |
Different Alteration |
Crizotinib (ALK inhibitor) |
Responsive |
IM |
Case report |
| ROS1 |
intron_variant), ROS1 MUT* (intron_variant |
Different Alteration |
Crizotinib (ALK inhibitor) |
Responsive |
NSCLC |
FDA guidelines |
| ROS1 |
intron_variant), ROS1 MUT* (intron_variant |
Different Alteration |
Cabozantinib (Pan-kinase inhibitor) |
Responsive |
LUAD |
Case report |
| ROS1 |
intron_variant), ROS1 MUT* (intron_variant |
Different Alteration |
HSP90 inhibitor |
Responsive |
LUAD |
Pre-clinical |
| ROS1 |
intron_variant), ROS1 MUT* (intron_variant |
Different Alteration |
Crizotinib (ALK inhibitor) |
Responsive |
LUAD |
Early trials |
| MET |
intron_variant), MET MUT* (intron_variant), MET MUT* (intron_variant |
Different Alteration |
Crizotinib (ALK inhibitor) |
Responsive |
G |
Case report |
| NTRK3 |
intron_variant), NTRK3 MUT* (intron_variant), NTRK3 MUT* (intron_variant |
Different Alteration |
IGF1R inhibitor |
Responsive |
BRCA |
Pre-clinical |
| NTRK3 |
intron_variant), NTRK3 MUT* (intron_variant), NTRK3 MUT* (intron_variant |
Different Alteration |
PI3K pathway inhibitor |
Responsive |
BRCA |
Pre-clinical |
| NTRK3 |
intron_variant), NTRK3 MUT* (intron_variant), NTRK3 MUT* (intron_variant |
Different Alteration |
Midostaurin (Pan-TK inhibitor) |
Responsive |
BRCA |
Pre-clinical |
| NTRK1 |
intron_variant |
Different Alteration |
Crizotinib (ALK inhibitor) |
Responsive |
LUAD |
Case report |
| NTRK1 |
intron_variant |
Different Alteration |
Pan-TKR inhibitor |
Responsive |
CANCER |
Early trials |
| NTRK1 |
intron_variant |
Different Alteration |
Pan-TK inhibitor |
Responsive |
LUAD |
Pre-clinical |
| NTRK1 |
intron_variant |
Different Alteration |
Pan-TK inhibitor |
Responsive |
COREAD |
Case report |
| NRG1 |
intron_variant), NRG1 MUT* (intron_variant), NRG1 MUT* (intron_variant), NRG1 MUT* (intron_variant), NRG1 MUT* (intron_variant |
Different Alteration |
Lapatinib (ERBB2 inhibitor) |
Responsive |
LUAD |
Pre-clinical |
| NRG1 |
intron_variant), NRG1 MUT* (intron_variant), NRG1 MUT* (intron_variant), NRG1 MUT* (intron_variant), NRG1 MUT* (intron_variant |
Different Alteration |
Afatinib (ERBB2 & EGFR inhibitor) |
Responsive |
LUAD |
Case report |
| BRD4 |
intron_variant |
Different Alteration |
BET inhibitor |
Responsive |
NMC |
Case report |
| BCR |
intron_variant |
Different Alteration |
Ponatinib (BCR-ABL inhibitor & Pan-TK inhibitor) |
Responsive |
CML, ALL |
FDA guidelines |
| BCR |
intron_variant |
Different Alteration |
Imatinib (BCR-ABL inhibitor & KIT inhibitor) |
Responsive |
ALL, CML |
FDA guidelines |
| BCR |
intron_variant |
Different Alteration |
Nilotinib (BCR-ABL inhibitor) |
Responsive |
CML |
FDA guidelines |
| BCR |
intron_variant |
Different Alteration |
Bosutinib (BCR-ABL inhibitor) |
Responsive |
CML |
FDA guidelines |
| BCR |
intron_variant |
Different Alteration |
Dasatinib;Venetoclax (BCR-ABL inhibitor;BCL2 inhibitor) |
Responsive |
ALL |
Pre-clinical |
| BCR |
intron_variant |
Different Alteration |
Dasatinib (BCR-ABL inhibitor) |
Responsive |
ALL, CML |
FDA guidelines |
| ALK |
intron_variant), ALK MUT* (intron_variant |
Different Alteration |
ALK inhibitor;SRC inhibitor |
Responsive |
LUAD |
Pre-clinical |
| ALK |
intron_variant), ALK MUT* (intron_variant |
Different Alteration |
Crizotinib (ALK inhibitor) |
Responsive |
GB, LY |
Early trials |
| ALK |
intron_variant), ALK MUT* (intron_variant |
Different Alteration |
Lorlatinib (ALK&ROS1 inhibitor) |
Responsive |
NSCLC |
Early trials |
| ALK |
intron_variant), ALK MUT* (intron_variant |
Different Alteration |
Crizotinib (ALK inhibitor) |
Responsive |
THCA, IM |
Case report |
| ALK |
intron_variant), ALK MUT* (intron_variant |
Different Alteration |
HSP90 inhibitor |
Responsive |
LUAD |
Early trials |
| ALK |
intron_variant), ALK MUT* (intron_variant |
Different Alteration |
Ceritinib (ALK inhibitor) |
Responsive |
LUAD, NSCLC |
FDA guidelines |
| ALK |
intron_variant), ALK MUT* (intron_variant |
Different Alteration |
Ceritinib (ALK inhibitor) |
Responsive |
IM, COREAD |
Case report |
| ALK |
intron_variant), ALK MUT* (intron_variant |
Different Alteration |
Crizotinib (ALK inhibitor) |
Responsive |
LUAD, NSCLC |
FDA guidelines |
| ALK |
intron_variant), ALK MUT* (intron_variant |
Different Alteration |
Brigatinib (Pan-TK inhibitor) |
Responsive |
NSCLC |
FDA guidelines |
| ALK |
intron_variant), ALK MUT* (intron_variant |
Different Alteration |
Entrictinib (Pan-TK inhibitor) |
Responsive |
COREAD |
Case report |
| ALK |
intron_variant), ALK MUT* (intron_variant |
Different Alteration |
Alectinib (ALK inhibitor) |
Responsive |
LUAD |
FDA guidelines |
| ALK |
intron_variant), ALK MUT* (intron_variant |
Different Alteration |
HSP90 inhibitor (HSP90 inhibitor) |
Responsive |
LUAD |
Early trials |
| ALK |
intron_variant), ALK MUT* (intron_variant |
Different Alteration |
ALK inhibitor;IGF1R inhibitor |
Responsive |
LUAD |
Pre-clinical |
| ALK |
intron_variant), ALK MUT* (intron_variant |
Different Alteration |
ALK inhibitor;MEK inhibitor |
Responsive |
LUAD |
Pre-clinical |
| ALK |
intron_variant), ALK MUT* (intron_variant |
Different Alteration |
novel ALK inhibitor |
Responsive |
LUAD |
Early trials |
| ALK |
intron_variant), ALK MUT* (intron_variant |
Different Alteration |
Ceritinib (ALK inhibitor) |
Responsive |
HEMATO |
Early trials |
| ALK |
intron_variant), ALK MUT* (intron_variant |
Different Alteration |
ALK inhibitor |
Responsive |
COREAD |
Case report |
| ALK |
intron_variant), ALK MUT* (intron_variant |
Different Alteration |
Alectinib (ALK inhibitor) |
Responsive |
NSCLC |
FDA guidelines |
| ALK |
intron_variant), ALK MUT* (intron_variant |
Different Alteration |
MEK inhibitor (ALK inhibitor;MEK inhibitor) |
Responsive |
LUAD |
Pre-clinical |
| ROS1 |
intron_variant |
Different Alteration |
Crizotinib (ALK inhibitor) |
Responsive |
IM |
Case report |
| ROS1 |
intron_variant |
Different Alteration |
Crizotinib (ALK inhibitor) |
Responsive |
NSCLC |
FDA guidelines |
| ROS1 |
intron_variant |
Different Alteration |
Cabozantinib (Pan-kinase inhibitor) |
Responsive |
LUAD |
Case report |
| ROS1 |
intron_variant |
Different Alteration |
HSP90 inhibitor |
Responsive |
LUAD |
Pre-clinical |
| ROS1 |
intron_variant |
Different Alteration |
Crizotinib (ALK inhibitor) |
Responsive |
LUAD |
Early trials |
| NTRK3 |
intron_variant |
Different Alteration |
IGF1R inhibitor |
Responsive |
BRCA |
Pre-clinical |
| NTRK3 |
intron_variant |
Different Alteration |
PI3K pathway inhibitor |
Responsive |
BRCA |
Pre-clinical |
| NTRK3 |
intron_variant |
Different Alteration |
Midostaurin (Pan-TK inhibitor) |
Responsive |
BRCA |
Pre-clinical |
| ERBB4 |
intron_variant), ERBB4 MUT* (intron_variant), ERBB4 MUT* (intron_variant |
Different Alteration |
Lapatinib;Afatinib (ERBB2 inhibitor;ERBB2 & EGFR inhibitor) |
Responsive |
LUAD |
Pre-clinical |
| EGFR |
intron_variant), EGFR MUT* (intron_variant |
Different Alteration |
Erlotinib (EGFR inhibitor) |
Responsive |
L |
Case report |
| EGFR |
intron_variant), EGFR MUT* (intron_variant |
Different Alteration |
Erlotinib (EGFR inhibitor) |
Responsive |
NSCLC |
Case report |
| ALK |
intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant |
Different Alteration |
ALK inhibitor;SRC inhibitor |
Responsive |
LUAD |
Pre-clinical |
| ALK |
intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant |
Different Alteration |
Crizotinib (ALK inhibitor) |
Responsive |
GB, LY |
Early trials |
| ALK |
intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant |
Different Alteration |
Lorlatinib (ALK&ROS1 inhibitor) |
Responsive |
NSCLC |
Early trials |
| ALK |
intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant |
Different Alteration |
Crizotinib (ALK inhibitor) |
Responsive |
THCA, IM |
Case report |
| ALK |
intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant |
Different Alteration |
HSP90 inhibitor |
Responsive |
LUAD |
Early trials |
| ALK |
intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant |
Different Alteration |
Ceritinib (ALK inhibitor) |
Responsive |
LUAD, NSCLC |
FDA guidelines |
| ALK |
intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant |
Different Alteration |
Ceritinib (ALK inhibitor) |
Responsive |
IM, COREAD |
Case report |
| ALK |
intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant |
Different Alteration |
Crizotinib (ALK inhibitor) |
Responsive |
LUAD, NSCLC |
FDA guidelines |
| ALK |
intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant |
Different Alteration |
Brigatinib (Pan-TK inhibitor) |
Responsive |
NSCLC |
FDA guidelines |
| ALK |
intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant |
Different Alteration |
Entrictinib (Pan-TK inhibitor) |
Responsive |
COREAD |
Case report |
| ALK |
intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant |
Different Alteration |
Alectinib (ALK inhibitor) |
Responsive |
LUAD |
FDA guidelines |
| ALK |
intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant |
Different Alteration |
HSP90 inhibitor (HSP90 inhibitor) |
Responsive |
LUAD |
Early trials |
| ALK |
intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant |
Different Alteration |
ALK inhibitor;IGF1R inhibitor |
Responsive |
LUAD |
Pre-clinical |
| ALK |
intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant |
Different Alteration |
ALK inhibitor;MEK inhibitor |
Responsive |
LUAD |
Pre-clinical |
| ALK |
intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant |
Different Alteration |
novel ALK inhibitor |
Responsive |
LUAD |
Early trials |
| ALK |
intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant |
Different Alteration |
Ceritinib (ALK inhibitor) |
Responsive |
HEMATO |
Early trials |
| ALK |
intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant |
Different Alteration |
ALK inhibitor |
Responsive |
COREAD |
Case report |
| ALK |
intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant |
Different Alteration |
Alectinib (ALK inhibitor) |
Responsive |
NSCLC |
FDA guidelines |
| ALK |
intron_variant), ALK MUT* (intron_variant), ALK MUT* (intron_variant |
Different Alteration |
MEK inhibitor (ALK inhibitor;MEK inhibitor) |
Responsive |
LUAD |
Pre-clinical |
| AKT3 |
intron_variant), AKT3 MUT* (intron_variant), AKT3 MUT* (intron_variant), AKT3 MUT* (intron_variant), AKT3 MUT* (intron_variant |
Different Alteration |
ATP competitive AKT inhibitor |
Responsive |
BRCA |
Pre-clinical |
| ERBB4 |
3-UTRSNV |
Different Alteration |
Lapatinib;Afatinib (ERBB2 inhibitor;ERBB2 & EGFR inhibitor) |
Responsive |
LUAD |
Pre-clinical |
| JAK2 |
intron_variant |
Different Alteration |
Ruxolitinib (JAK inhibitor) |
Responsive |
ALL |
Pre-clinical |
| ESR1 |
intron_variant), ESR1 MUT* (intron_variant |
Different Alteration |
ESR1 inhibitor |
Resistant |
BRCA |
Pre-clinical |
| ERBB4 |
intron_variant), ERBB4 MUT* (intron_variant), ERBB4 MUT* (intron_variant), ERBB4 MUT* (intron_variant), ERBB4 MUT* (intron_variant |
Different Alteration |
Lapatinib;Afatinib (ERBB2 inhibitor;ERBB2 & EGFR inhibitor) |
Responsive |
LUAD |
Pre-clinical |
| FGFR2 |
intron_variant), FGFR2 MUT* (intron_variant |
Different Alteration |
FGFR inhibitor |
Responsive |
COREAD |
Early trials |
| FGFR2 |
intron_variant), FGFR2 MUT* (intron_variant |
Different Alteration |
FGFR inhibitor |
Responsive |
BT |
Early trials |
| RAF1 |
intron_variant |
Different Alteration |
Sorafenib (Pan-TK inhibitor) |
Responsive |
PRAD |
Pre-clinical |
| RAF1 |
intron_variant |
Different Alteration |
Pan-RAF inhibitor |
Responsive |
PRAD |
Pre-clinical |
| RAF1 |
intron_variant |
Different Alteration |
U0126 (MEK inhibitor) |
Responsive |
PRAD |
Pre-clinical |
| KMT2A |
intron_variant), MLL MUT* (intron_variant |
Different Alteration |
EPZ-5676 (DOT1L inhibitor) |
Responsive |
ALL, MDS, AML |
Early trials |
| KMT2A |
intron_variant), MLL MUT* (intron_variant |
Different Alteration |
BET inhibitor |
Responsive |
ALL |
Pre-clinical |
| KMT2A |
intron_variant), MLL MUT* (intron_variant |
Different Alteration |
BET inhibitor |
Responsive |
AML |
Pre-clinical |
| KMT2A |
intron_variant), MLL MUT* (intron_variant |
Different Alteration |
Daunorubicin (Chemotherapy) |
Responsive |
AML |
FDA guidelines |
| KMT2A |
intron_variant), MLL MUT* (intron_variant |
Different Alteration |
Belinostat (HDAC inhibitor) |
Responsive |
AML |
Early trials |
| KMT2A |
intron_variant), MLL MUT* (intron_variant |
Different Alteration |
DOT1L inhibitor |
Responsive |
AML |
Early trials |
| NOTCH2 |
intron_variant |
Different Alteration |
Gamma secretase inhibitor |
Responsive |
BRCA |
Pre-clinical |
| ERBB4 |
intron_variant), ERBB4 MUT* (intron_variant), ERBB4 MUT* (intron_variant), ERBB4 MUT* (intron_variant), ERBB4 MUT* (intron_variant), ERBB4 MUT* (intron_variant |
Different Alteration |
Lapatinib;Afatinib (ERBB2 inhibitor;ERBB2 & EGFR inhibitor) |
Responsive |
LUAD |
Pre-clinical |
| ALK |
intron_variant |
Different Alteration |
ALK inhibitor;SRC inhibitor |
Responsive |
LUAD |
Pre-clinical |
| ALK |
intron_variant |
Different Alteration |
Crizotinib (ALK inhibitor) |
Responsive |
GB, LY |
Early trials |
| ALK |
intron_variant |
Different Alteration |
Lorlatinib (ALK&ROS1 inhibitor) |
Responsive |
NSCLC |
Early trials |
| ALK |
intron_variant |
Different Alteration |
Crizotinib (ALK inhibitor) |
Responsive |
THCA, IM |
Case report |
| ALK |
intron_variant |
Different Alteration |
HSP90 inhibitor |
Responsive |
LUAD |
Early trials |
| ALK |
intron_variant |
Different Alteration |
Ceritinib (ALK inhibitor) |
Responsive |
LUAD, NSCLC |
FDA guidelines |
| ALK |
intron_variant |
Different Alteration |
Ceritinib (ALK inhibitor) |
Responsive |
IM, COREAD |
Case report |
| ALK |
intron_variant |
Different Alteration |
Crizotinib (ALK inhibitor) |
Responsive |
LUAD, NSCLC |
FDA guidelines |
| ALK |
intron_variant |
Different Alteration |
Brigatinib (Pan-TK inhibitor) |
Responsive |
NSCLC |
FDA guidelines |
| ALK |
intron_variant |
Different Alteration |
Entrictinib (Pan-TK inhibitor) |
Responsive |
COREAD |
Case report |
| ALK |
intron_variant |
Different Alteration |
Alectinib (ALK inhibitor) |
Responsive |
LUAD |
FDA guidelines |
| ALK |
intron_variant |
Different Alteration |
HSP90 inhibitor (HSP90 inhibitor) |
Responsive |
LUAD |
Early trials |
| ALK |
intron_variant |
Different Alteration |
ALK inhibitor;IGF1R inhibitor |
Responsive |
LUAD |
Pre-clinical |
| ALK |
intron_variant |
Different Alteration |
ALK inhibitor;MEK inhibitor |
Responsive |
LUAD |
Pre-clinical |
| ALK |
intron_variant |
Different Alteration |
novel ALK inhibitor |
Responsive |
LUAD |
Early trials |
| ALK |
intron_variant |
Different Alteration |
Ceritinib (ALK inhibitor) |
Responsive |
HEMATO |
Early trials |
| ALK |
intron_variant |
Different Alteration |
ALK inhibitor |
Responsive |
COREAD |
Case report |
| ALK |
intron_variant |
Different Alteration |
Alectinib (ALK inhibitor) |
Responsive |
NSCLC |
FDA guidelines |
| ALK |
intron_variant |
Different Alteration |
MEK inhibitor (ALK inhibitor;MEK inhibitor) |
Responsive |
LUAD |
Pre-clinical |
| KMT2A |
intron_variant |
Different Alteration |
EPZ-5676 (DOT1L inhibitor) |
Responsive |
ALL, MDS, AML |
Early trials |
| KMT2A |
intron_variant |
Different Alteration |
BET inhibitor |
Responsive |
ALL |
Pre-clinical |
| KMT2A |
intron_variant |
Different Alteration |
BET inhibitor |
Responsive |
AML |
Pre-clinical |
| KMT2A |
intron_variant |
Different Alteration |
Daunorubicin (Chemotherapy) |
Responsive |
AML |
FDA guidelines |
| KMT2A |
intron_variant |
Different Alteration |
Belinostat (HDAC inhibitor) |
Responsive |
AML |
Early trials |
| KMT2A |
intron_variant |
Different Alteration |
DOT1L inhibitor |
Responsive |
AML |
Early trials |
| NOTCH2 |
intron_variant), NOTCH2 MUT* (intron_variant), NOTCH2 MUT* (intron_variant |
Different Alteration |
Gamma secretase inhibitor |
Responsive |
BRCA |
Pre-clinical |
| NRG1 |
M471T |
Different Alteration |
Lapatinib (ERBB2 inhibitor) |
Responsive |
LUAD |
Pre-clinical |
| NRG1 |
M471T |
Different Alteration |
Afatinib (ERBB2 & EGFR inhibitor) |
Responsive |
LUAD |
Case report |
| NRG1 |
intron_variant), NRG1 MUT* (intron_variant |
Different Alteration |
Lapatinib (ERBB2 inhibitor) |
Responsive |
LUAD |
Pre-clinical |
| NRG1 |
intron_variant), NRG1 MUT* (intron_variant |
Different Alteration |
Afatinib (ERBB2 & EGFR inhibitor) |
Responsive |
LUAD |
Case report |
| AKT3 |
intron_variant |
Different Alteration |
ATP competitive AKT inhibitor |
Responsive |
BRCA |
Pre-clinical |
| VHL |
splice_donor_variant |
Complete Match |
VEGFR inhibitor |
Responsive |
R |
Pre-clinical |
| ERBB4 |
intron_variant), ERBB4 MUT* (intron_variant), ERBB4 MUT* (intron_variant), ERBB4 MUT* (intron_variant |
Different Alteration |
Lapatinib;Afatinib (ERBB2 inhibitor;ERBB2 & EGFR inhibitor) |
Responsive |
LUAD |
Pre-clinical |
| ARID1A |
Q2100* |
Complete Match |
EZH2 inhibitor |
Responsive |
OV |
Pre-clinical |
| ARID1A |
Q2100* |
Complete Match |
PD1 inhibitor |
Responsive |
OV |
Pre-clinical |
| ARID1A |
Q2100* |
Complete Match |
PARP inhibitor |
Responsive |
CANCER |
Pre-clinical |
| ARID1A |
Q2100* |
Complete Match |
ATR inhibitor |
Responsive |
CANCER |
Pre-clinical |
| NTRK3 |
intron_variant), NTRK3 MUT* (intron_variant |
Different Alteration |
IGF1R inhibitor |
Responsive |
BRCA |
Pre-clinical |
| NTRK3 |
intron_variant), NTRK3 MUT* (intron_variant |
Different Alteration |
PI3K pathway inhibitor |
Responsive |
BRCA |
Pre-clinical |
| NTRK3 |
intron_variant), NTRK3 MUT* (intron_variant |
Different Alteration |
Midostaurin (Pan-TK inhibitor) |
Responsive |
BRCA |
Pre-clinical |
| PML |
intron_variant |
Different Alteration |
Volasertib (PLK1 inhibitor) |
Responsive |
AML |
Early trials |
| PML |
intron_variant |
Different Alteration |
Tretinoin (Retinoid) |
Responsive |
APML |
FDA guidelines |
| PML |
intron_variant |
Different Alteration |
Tretinoin;Arsenic Trioxide (Retinoid;Chemotherapy) |
Responsive |
AML |
FDA guidelines |
| TSC2 |
splice_acceptor_variant |
Different Mutation |
Temsirolimus (MTOR inhibitor) |
Responsive |
ED |
Case report |
| TSC2 |
splice_acceptor_variant |
Complete Match |
Everolimus (MTOR inhibitor) |
Responsive |
RA, GCA |
FDA guidelines |
| TSC2 |
splice_acceptor_variant |
Complete Match |
SRC inhibitor |
Responsive |
LAM |
Pre-clinical |
| TSC2 |
splice_acceptor_variant |
Complete Match |
Everolimus (MTOR inhibitor) |
Responsive |
BLCA |
Early trials |
| TSC2 |
splice_acceptor_variant |
Complete Match |
MTOR inhibitor |
Responsive |
RA |
Early trials |
| TSC2 |
splice_acceptor_variant |
Different Mutation |
Everolimus (MTOR inhibitor) |
Responsive |
THCA |
Case report |
| TSC2 |
splice_acceptor_variant |
Different Alteration |
SRC inhibitor |
Responsive |
LAM |
Pre-clinical |
| JAK2 |
intron_variant), JAK2 MUT* (intron_variant |
Different Alteration |
Ruxolitinib (JAK inhibitor) |
Responsive |
ALL |
Pre-clinical |
| SETD2 |
Q2329* |
Complete Match |
WEE1 inhibitor |
Responsive |
CANCER |
Pre-clinical |
| SETD2 |
Q2329* |
Different Alteration |
WEE1 inhibitor |
Responsive |
CANCER |
Pre-clinical |
| FGFR3 |
intron_variant |
Different Alteration |
FGFR inhibitor |
Responsive |
BLCA |
Case report |
| FGFR3 |
intron_variant |
Different Alteration |
FGFR inhibitor |
Responsive |
G |
Early trials |
| FGFR3 |
intron_variant |
Different Alteration |
FGFR inhibitor |
Responsive |
NSCLC |
Pre-clinical |
| NOTCH1 |
intron_variant |
Different Alteration |
Gamma secretase inhibitor |
Responsive |
ALL |
Pre-clinical |
| NOTCH1 |
intron_variant |
Different Alteration |
Gamma secretase inhibitor |
Responsive |
BRCA |
Pre-clinical |
| CDKN1A |
CdsStartSNV |
Complete Match |
CDK2/4 inhibitor |
Responsive |
CANCER |
Pre-clinical |
| CDKN1A |
CdsStartSNV |
Different Alteration |
CDK2/4 inhibitor |
Responsive |
CANCER |
Pre-clinical |
| NOTCH1 |
intron_variant), NOTCH1 MUT* (intron_variant), NOTCH1 MUT* (R1683Q |
Different Alteration |
Gamma secretase inhibitor |
Responsive |
ALL |
Pre-clinical |
| NOTCH1 |
intron_variant), NOTCH1 MUT* (intron_variant), NOTCH1 MUT* (R1683Q |
Different Alteration |
Gamma secretase inhibitor |
Responsive |
BRCA |
Pre-clinical |
| NRG1 |
intron_variant), NRG1 MUT* (intron_variant), NRG1 MUT* (intron_variant |
Different Alteration |
Lapatinib (ERBB2 inhibitor) |
Responsive |
LUAD |
Pre-clinical |
| NRG1 |
intron_variant), NRG1 MUT* (intron_variant), NRG1 MUT* (intron_variant |
Different Alteration |
Afatinib (ERBB2 & EGFR inhibitor) |
Responsive |
LUAD |
Case report |
| MET |
T618T |
Different Alteration |
Crizotinib (ALK inhibitor) |
Responsive |
G |
Case report |
| RET |
intron_variant |
Different Alteration |
Nintedanib (Pan-TK inhibitor) |
Responsive |
LUAD |
Case report |
| RET |
intron_variant |
Different Alteration |
Cabozantinib (Pan-kinase inhibitor) |
Responsive |
LUAD |
Early trials |
| RET |
intron_variant |
Different Alteration |
RET inhibitor |
Responsive |
LUAD |
Pre-clinical |
| RET |
intron_variant |
Different Alteration |
Vandetanib (Pan-TK inhibitor) |
Responsive |
LUAD |
Early trials |
| RET |
intron_variant |
Different Alteration |
Sunitinib (Pan-TK inhibitor) |
Responsive |
LUAD |
Early trials |
| RET |
intron_variant |
Different Alteration |
Cabozantinib (Pan-kinase inhibitor) |
Responsive |
THCA |
Pre-clinical |
| RET |
intron_variant |
Different Alteration |
Vandetanib (Pan-TK inhibitor) |
Responsive |
THCA |
Pre-clinical |
| RET |
intron_variant |
Different Alteration |
Sunitinib (Pan-TK inhibitor) |
Responsive |
THCA |
Pre-clinical |
| RET |
intron_variant |
Different Alteration |
RET inhibitor |
Responsive |
TH |
Pre-clinical |
| PTEN |
G209* |
Different Mutation |
Cetuximab (EGFR mAb inhibitor) |
Resistant |
COREAD |
Case report |
| PTEN |
G209* |
Different Mutation |
Panitumumab (EGFR mAb inhibitor) |
Resistant |
COREAD |
Case report |
| PTEN |
G209* |
Different Mutation |
BYL719 (PIK3CA inhibitor) |
Resistant |
BRCA |
Case report |
| PTEN |
G209* |
Complete Match |
ATM inhibitor |
Responsive |
BRCA |
Pre-clinical |
| PTEN |
G209* |
Complete Match |
PI3K pathway inhibitor |
Responsive |
BRCA, ED, TH, CANCER, L, G, OV |
Pre-clinical |
| PTEN |
G209* |
Complete Match |
EGFR mAb inhibitor |
Resistant |
COREAD |
Late trials |
| PTEN |
G209* |
Complete Match |
Sirolimus (MTOR inhibitor) |
Responsive |
CANCER |
Early trials |
| PTEN |
G209* |
Complete Match |
PARP inhibitor |
Responsive |
ED |
Case report |
| PTEN |
G209* |
Complete Match |
PD1 Ab inhibitor |
Resistant |
CM |
Early trials |
| PTEN |
G209* |
Complete Match |
PI3K pathway inhibitor;AR antagonist |
Responsive |
PRAD |
Pre-clinical |
| PTEN |
G209* |
Complete Match |
Everolimus (MTOR inhibitor) |
Responsive |
PRAD |
Early trials |
| PTEN |
G209* |
Complete Match |
PI3K pathway inhibitor;MEK inhibitor |
Responsive |
OV |
Pre-clinical |
| PTEN |
G209* |
Complete Match |
PIK3CB inhibitor |
Responsive |
PRAD |
Case report |
| PTEN |
G209* |
Complete Match |
AKT inhibitor |
Responsive |
PA |
Case report |
| PTEN |
G209* |
Complete Match |
MTOR inhibitor |
No Responsive |
ED |
Early trials |
| PTEN |
G209* |
Different Alteration |
PI3K pathway inhibitor |
Responsive |
L, BRCA, CANCER, G, ED, TH, OV |
Pre-clinical |
| PTEN |
G209* |
Different Alteration |
Everolimus (MTOR inhibitor) |
Responsive |
PRAD |
Early trials |
| PTEN |
G209* |
Different Alteration |
Sirolimus (MTOR inhibitor) |
Responsive |
CANCER |
Early trials |
| PTEN |
G209* |
Different Alteration |
PARP inhibitor |
Responsive |
ED |
Case report |
| PTEN |
G209* |
Different Alteration |
PIK3CB inhibitor |
Responsive |
PRAD |
Case report |
| PTEN |
G209* |
Different Alteration |
EGFR mAb inhibitor |
Resistant |
COREAD |
Late trials |
| PTEN |
G209* |
Different Alteration |
MTOR inhibitor |
No Responsive |
ED |
Early trials |
| PTEN |
G209* |
Different Alteration |
AKT inhibitor |
Responsive |
PA |
Case report |
| PTEN |
G209* |
Different Alteration |
PI3K pathway inhibitor;AR antagonist |
Responsive |
PRAD |
Pre-clinical |
| PTEN |
G209* |
Different Alteration |
PI3K pathway inhibitor;MEK inhibitor |
Responsive |
OV |
Pre-clinical |
| ESR1 |
IntronicBlockSubstitution), ESR1 MUT* (intron_variant |
Different Alteration |
ESR1 inhibitor |
Resistant |
BRCA |
Pre-clinical |
| TP53 |
R280K |
Different Mutation |
HSP90 inhibitor |
Responsive |
CANCER |
Pre-clinical |
| TP53 |
R280K |
Complete Match |
Decitabine (Chemotherapy) |
Responsive |
AML, MDPS |
Early trials |
| TP53 |
R280K |
Complete Match |
Abemaciclib (CDK4/6 inhibitor) |
Resistant |
BRCA |
Early trials |
| TP53 |
R280K |
Different Mutation |
HDM2 inhibitor |
Responsive |
AML |
Early trials |
| TP53 |
R280K |
Complete Match |
Cisplatin (Chemotherapy) |
Resistant |
FGCT, MGCT |
Early trials |
| TP53 |
R280K |
Complete Match |
AZD6738 (ATR inhibitor) |
Responsive |
BCL |
Early trials |
| TP53 |
R280K |
Complete Match |
Gemcitabine (Chemotherapy) |
Responsive |
BLCA |
Pre-clinical |
| TP53 |
R280K |
Complete Match |
Mitomycin C (Chemotherapy) |
Responsive |
BLCA |
Pre-clinical |
| TP53 |
R280K |
Complete Match |
WEE1 inhibitor |
Responsive |
HNC |
Pre-clinical |
| TP53 |
R280K |
Complete Match |
MDM2 inhibitor |
Resistant |
LIP |
Early trials |
| TP53 |
R280K |
Complete Match |
Doxorubicin (Anthracycline antitumor antibiotic) |
Responsive |
BLCA |
Pre-clinical |
| TP53 |
R280K |
Complete Match |
Pramlintide (Amylin analogue) |
Responsive |
THYM |
Pre-clinical |
| TP53 |
R280K |
Complete Match |
WEE1 inhibitor |
Responsive |
OV |
Early trials |
| TP53 |
R280K |
Different Alteration |
Pramlintide (Amylin analogue) |
Responsive |
THYM |
Pre-clinical |
| TP53 |
R280K |
Different Alteration |
AZD6738 (ATR inhibitor) |
Responsive |
BCL |
Early trials |
| MET |
intron_variant), MET MUT* (intron_variant), MET MUT* (A320G), MET MUT* (intron_variant |
Different Alteration |
Crizotinib (ALK inhibitor) |
Responsive |
G |
Case report |
| NRG1 |
E478E), NRG1 MUT* (intron_variant), NRG1 MUT* (intron_variant |
Different Alteration |
Lapatinib (ERBB2 inhibitor) |
Responsive |
LUAD |
Pre-clinical |
| NRG1 |
E478E), NRG1 MUT* (intron_variant), NRG1 MUT* (intron_variant |
Different Alteration |
Afatinib (ERBB2 & EGFR inhibitor) |
Responsive |
LUAD |
Case report |
| PTEN |
S229*), PTEN MUT (splice_acceptor_variant |
Complete Match |
Cetuximab (EGFR mAb inhibitor) |
Resistant |
COREAD |
Case report |
| PTEN |
S229*), PTEN MUT (splice_acceptor_variant |
Complete Match |
Panitumumab (EGFR mAb inhibitor) |
Resistant |
COREAD |
Case report |
| PTEN |
S229*), PTEN MUT (splice_acceptor_variant |
Complete Match |
BYL719 (PIK3CA inhibitor) |
Resistant |
BRCA |
Case report |
| PTEN |
S229*), PTEN MUT (splice_acceptor_variant |
Complete Match |
ATM inhibitor |
Responsive |
BRCA |
Pre-clinical |
| PTEN |
S229*), PTEN MUT (splice_acceptor_variant |
Complete Match |
PI3K pathway inhibitor |
Responsive |
BRCA, ED, TH, CANCER, L, G, OV |
Pre-clinical |
| PTEN |
S229*), PTEN MUT (splice_acceptor_variant |
Complete Match |
EGFR mAb inhibitor |
Resistant |
COREAD |
Late trials |
| PTEN |
S229*), PTEN MUT (splice_acceptor_variant |
Complete Match |
Sirolimus (MTOR inhibitor) |
Responsive |
CANCER |
Early trials |
| PTEN |
S229*), PTEN MUT (splice_acceptor_variant |
Complete Match |
PARP inhibitor |
Responsive |
ED |
Case report |
| PTEN |
S229*), PTEN MUT (splice_acceptor_variant |
Complete Match |
PD1 Ab inhibitor |
Resistant |
CM |
Early trials |
| PTEN |
S229*), PTEN MUT (splice_acceptor_variant |
Complete Match |
PI3K pathway inhibitor;AR antagonist |
Responsive |
PRAD |
Pre-clinical |
| PTEN |
S229*), PTEN MUT (splice_acceptor_variant |
Complete Match |
Everolimus (MTOR inhibitor) |
Responsive |
PRAD |
Early trials |
| PTEN |
S229*), PTEN MUT (splice_acceptor_variant |
Complete Match |
PI3K pathway inhibitor;MEK inhibitor |
Responsive |
OV |
Pre-clinical |
| PTEN |
S229*), PTEN MUT (splice_acceptor_variant |
Complete Match |
PIK3CB inhibitor |
Responsive |
PRAD |
Case report |
| PTEN |
S229*), PTEN MUT (splice_acceptor_variant |
Complete Match |
AKT inhibitor |
Responsive |
PA |
Case report |
| PTEN |
S229*), PTEN MUT (splice_acceptor_variant |
Complete Match |
MTOR inhibitor |
No Responsive |
ED |
Early trials |
| PTEN |
S229*), PTEN MUT (splice_acceptor_variant |
Different Alteration |
PI3K pathway inhibitor |
Responsive |
L, BRCA, CANCER, G, ED, TH, OV |
Pre-clinical |
| PTEN |
S229*), PTEN MUT (splice_acceptor_variant |
Different Alteration |
Everolimus (MTOR inhibitor) |
Responsive |
PRAD |
Early trials |
| PTEN |
S229*), PTEN MUT (splice_acceptor_variant |
Different Alteration |
Sirolimus (MTOR inhibitor) |
Responsive |
CANCER |
Early trials |
| PTEN |
S229*), PTEN MUT (splice_acceptor_variant |
Different Alteration |
PARP inhibitor |
Responsive |
ED |
Case report |
| PTEN |
S229*), PTEN MUT (splice_acceptor_variant |
Different Alteration |
PIK3CB inhibitor |
Responsive |
PRAD |
Case report |
| PTEN |
S229*), PTEN MUT (splice_acceptor_variant |
Different Alteration |
EGFR mAb inhibitor |
Resistant |
COREAD |
Late trials |
| PTEN |
S229*), PTEN MUT (splice_acceptor_variant |
Different Alteration |
MTOR inhibitor |
No Responsive |
ED |
Early trials |
| PTEN |
S229*), PTEN MUT (splice_acceptor_variant |
Different Alteration |
AKT inhibitor |
Responsive |
PA |
Case report |
| PTEN |
S229*), PTEN MUT (splice_acceptor_variant |
Different Alteration |
PI3K pathway inhibitor;AR antagonist |
Responsive |
PRAD |
Pre-clinical |
| PTEN |
S229*), PTEN MUT (splice_acceptor_variant |
Different Alteration |
PI3K pathway inhibitor;MEK inhibitor |
Responsive |
OV |
Pre-clinical |



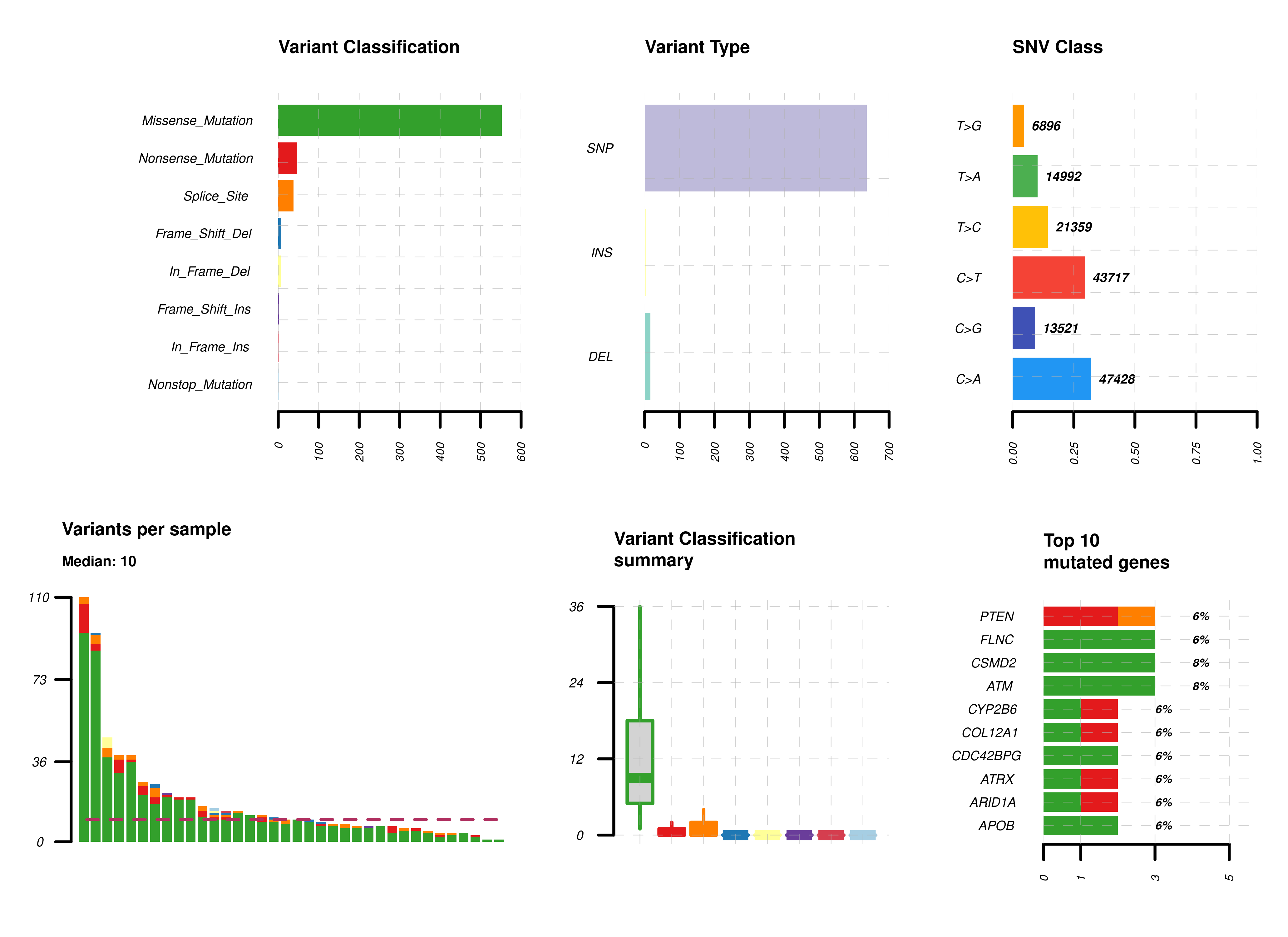
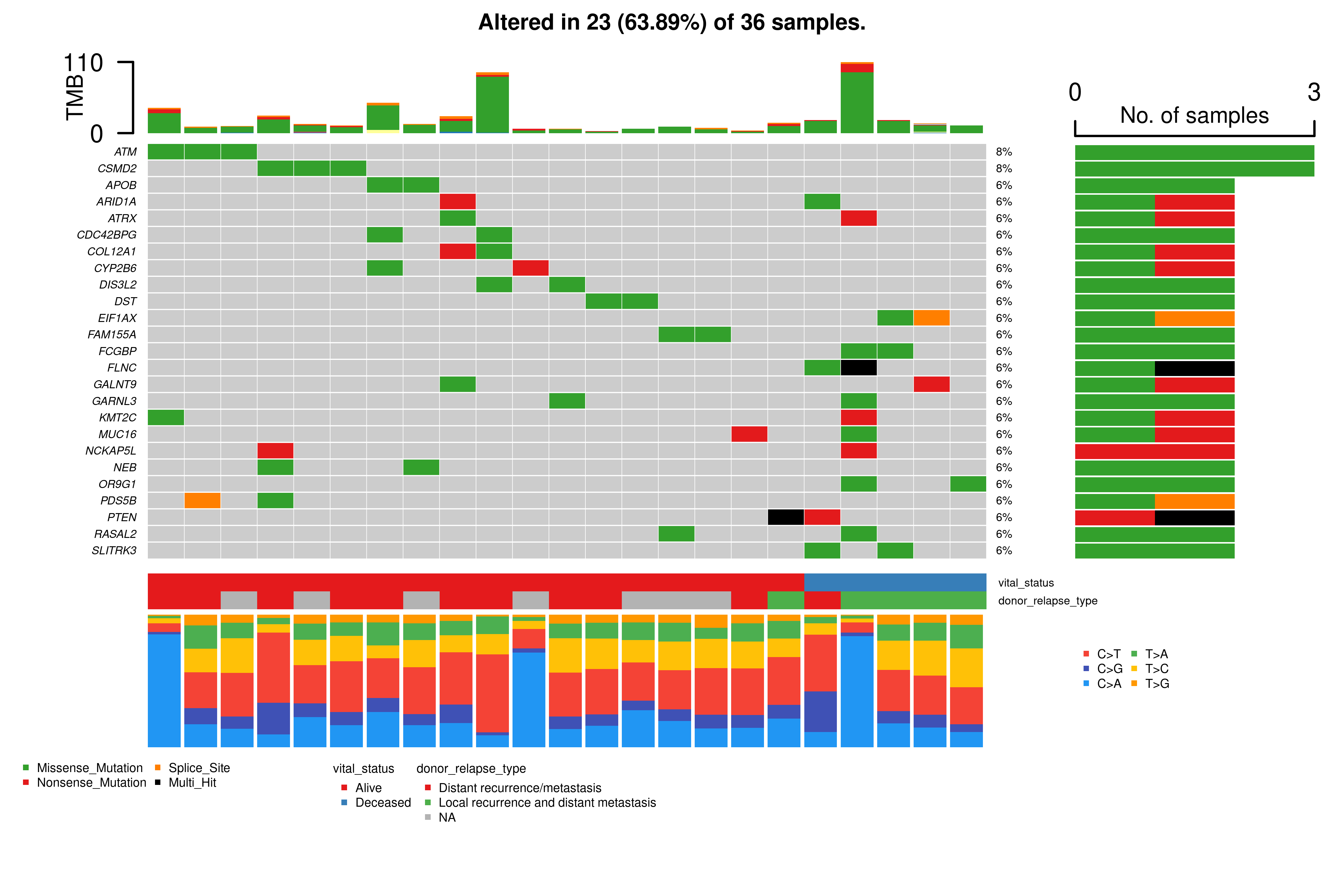
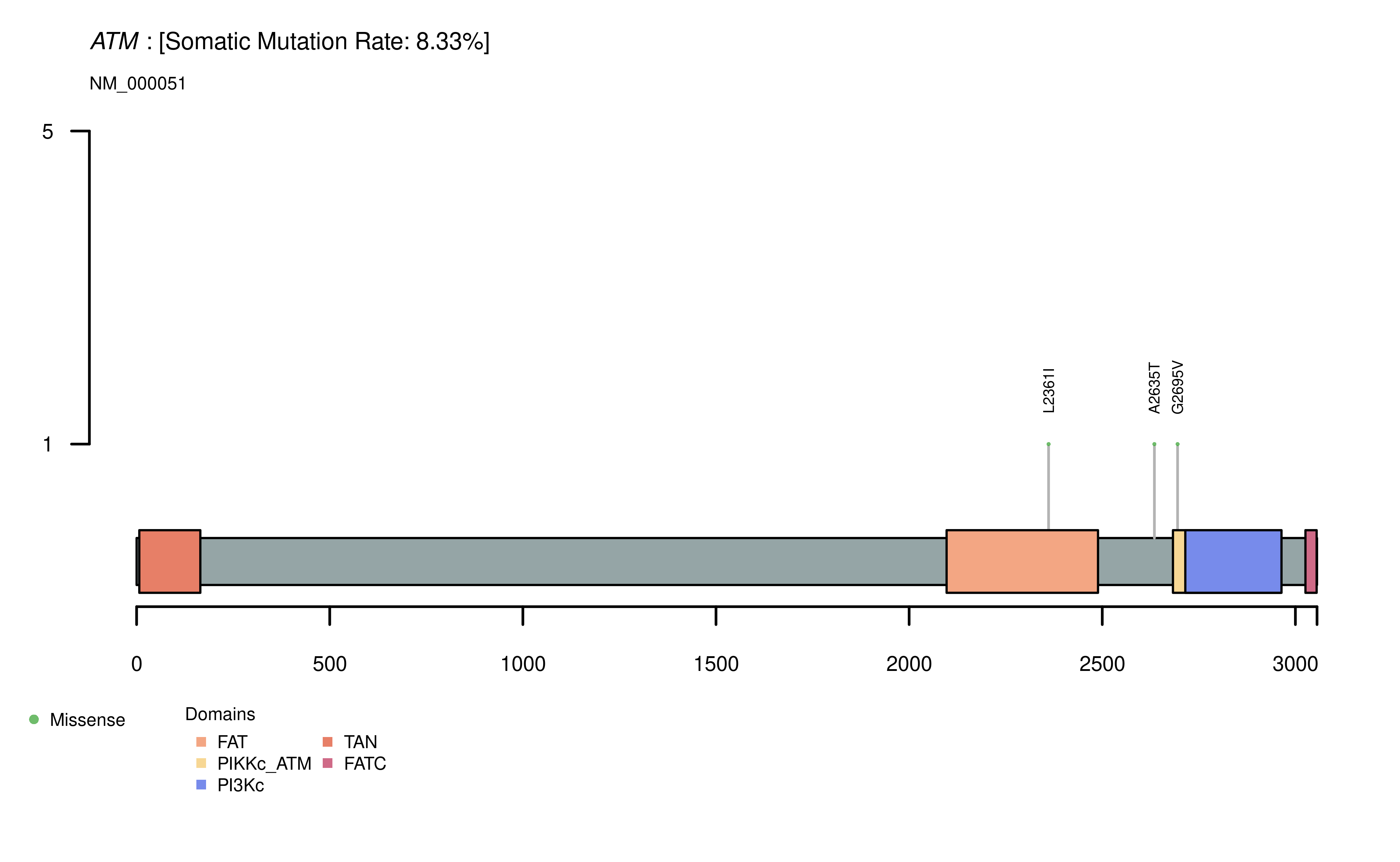
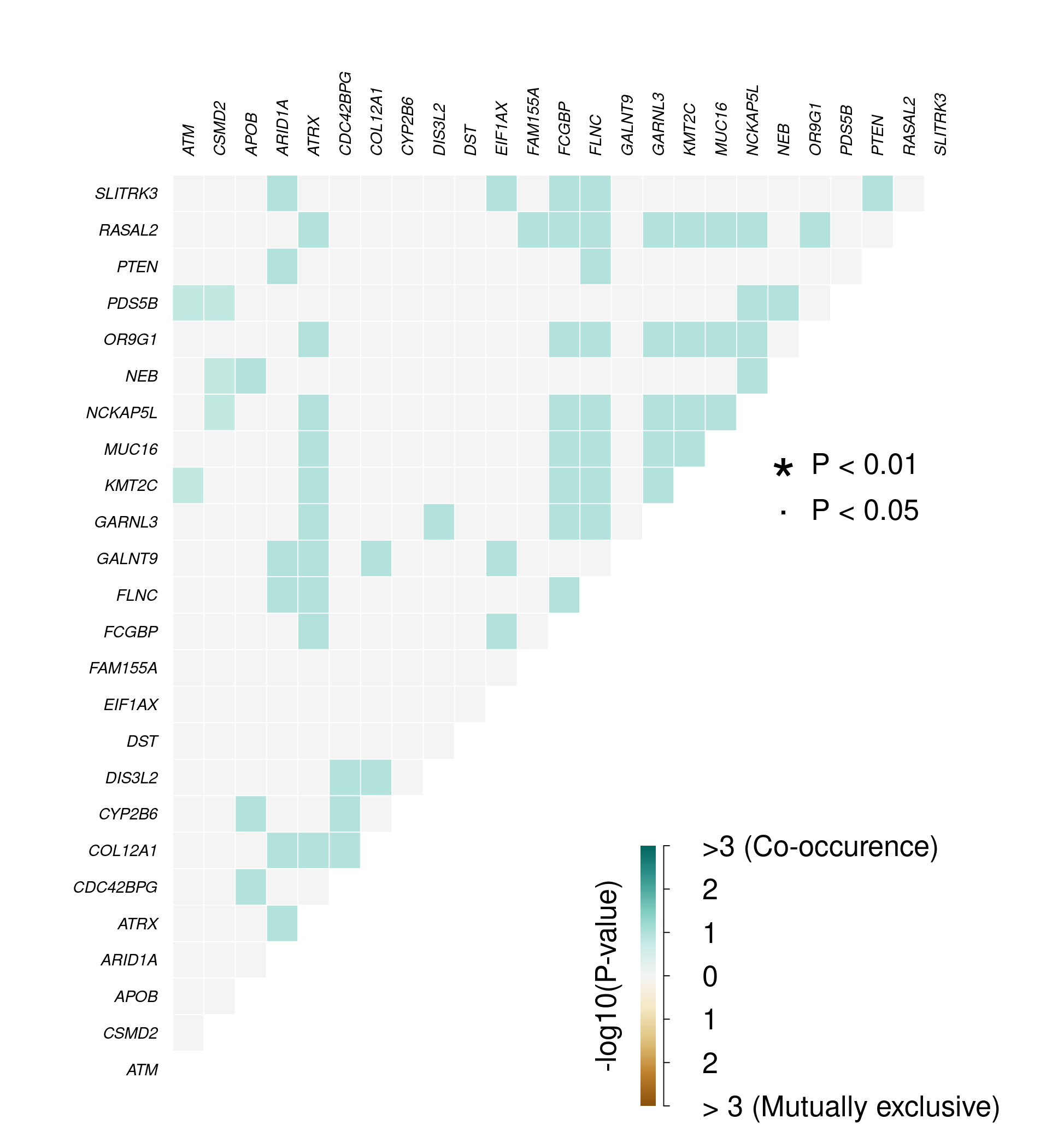
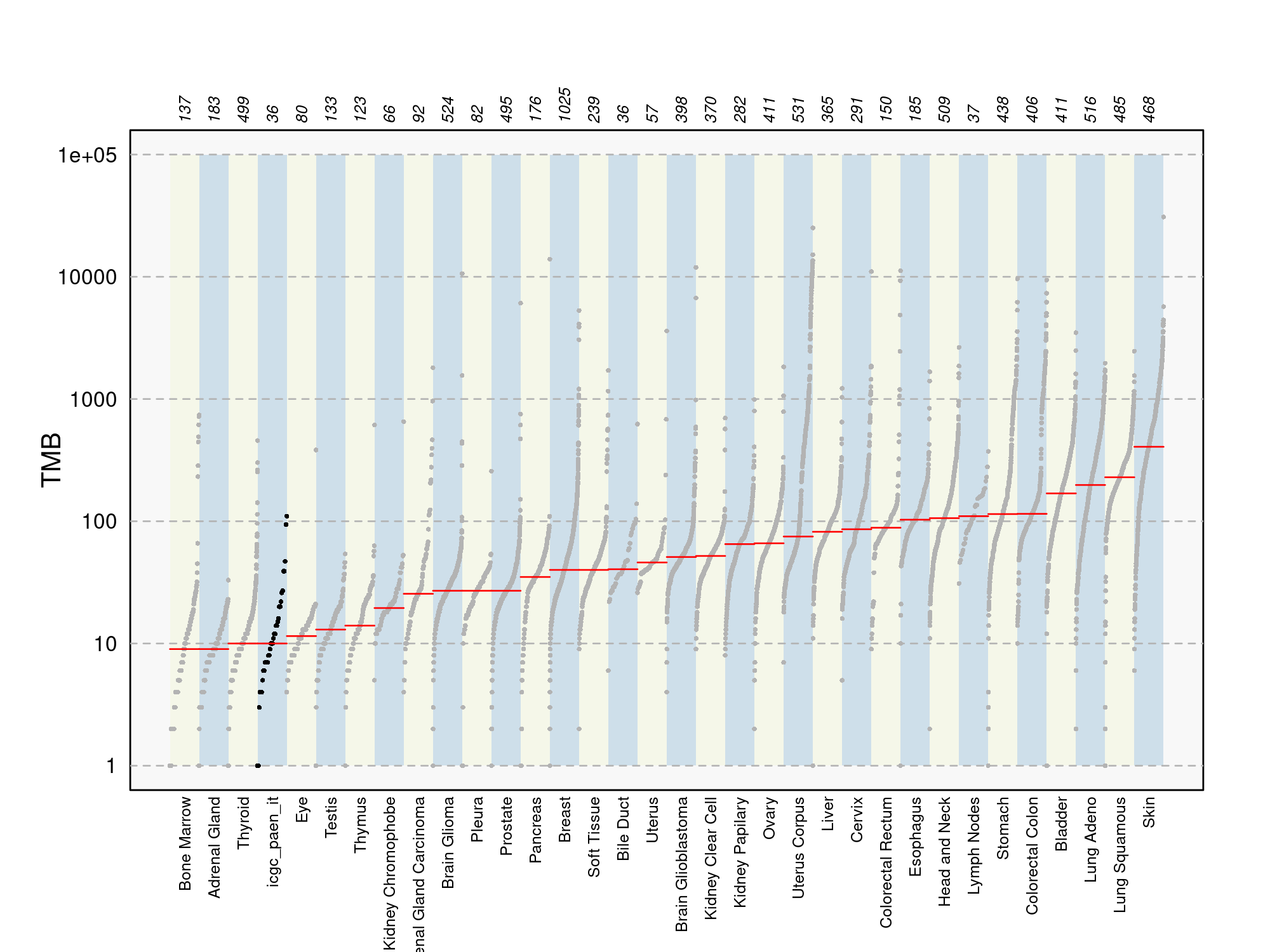
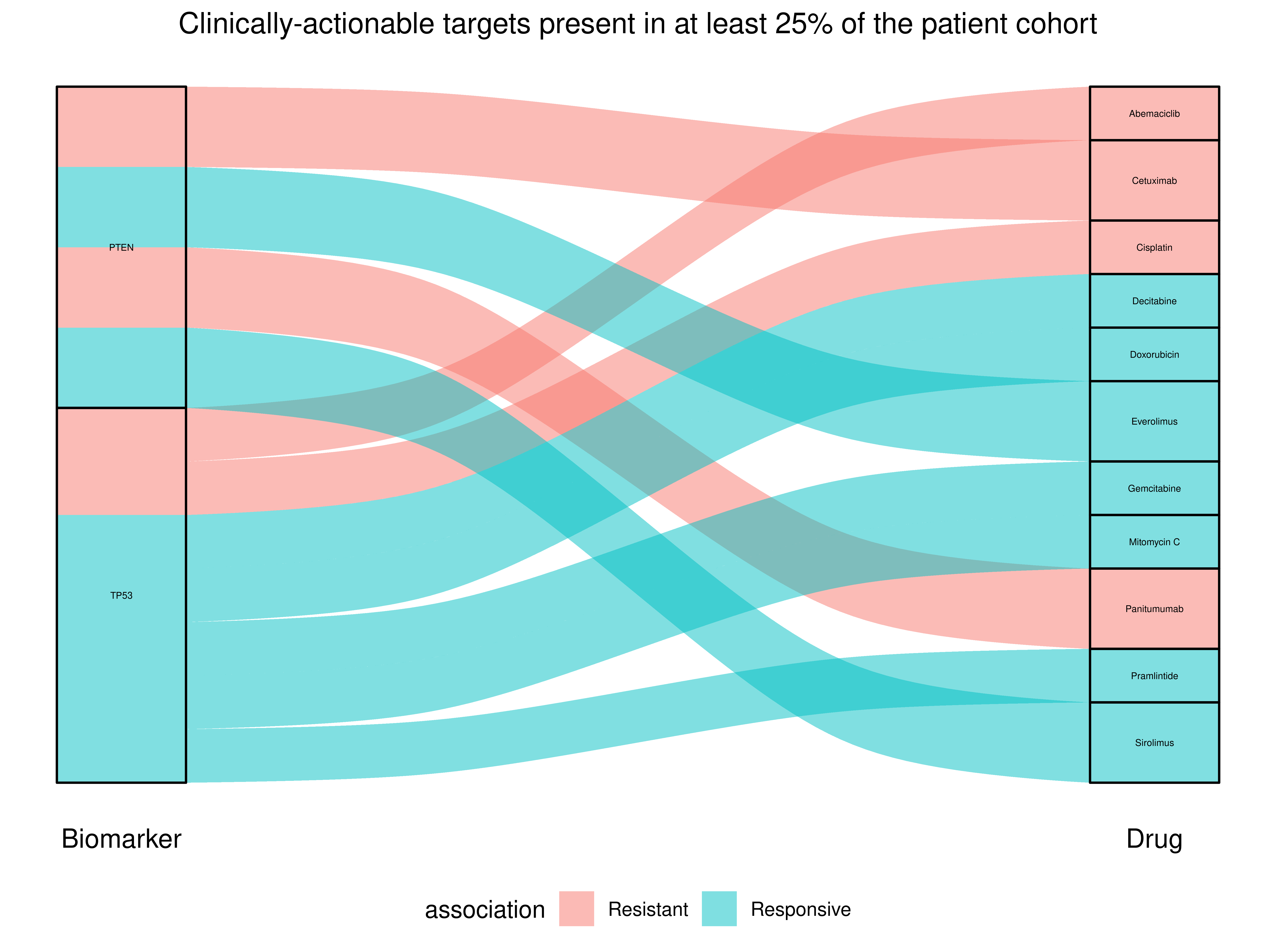
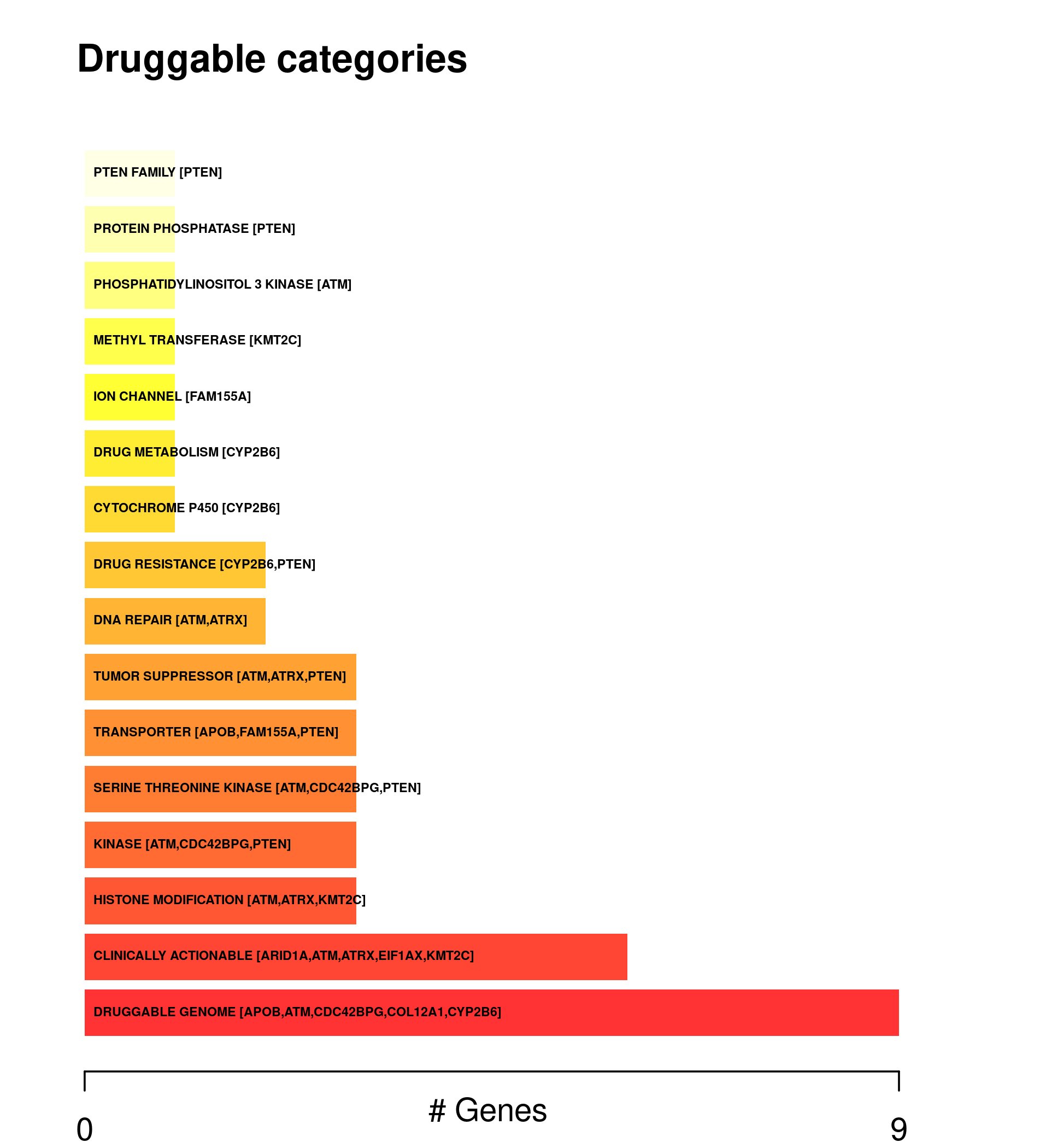
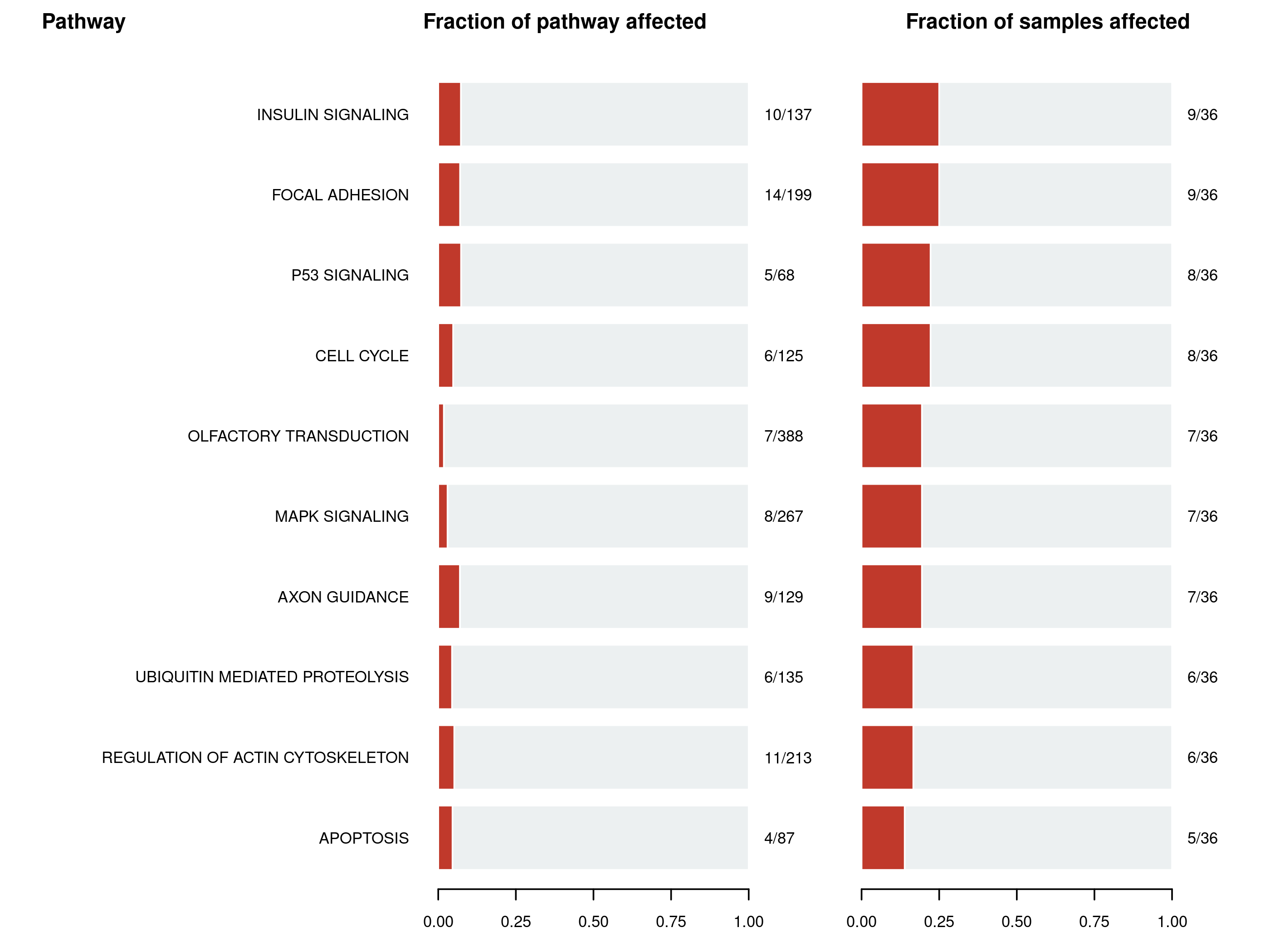

 Phenotype
Phenotype Protein
Protein Drug
Drug